lab starter - Virtual Homeschool Group
advertisement

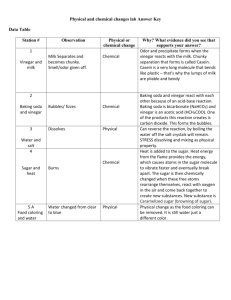
Moore, Timothy Moore, Timothy Instructor: Mrs. Tammy Moore Class: VHSG Online Chemistry 26 May 2009 EXPERIMENT 13.1 Determining the Change in Enthalpy of a Chemical Reaction To practice using calorimetry and discover how ΔH can be determined with an experiment. Abstract [What you will put here: Summarize the whole report in one, concise paragraph of about 100-200 words. You cannot write the abstract until after you've completed the report. Because it assumes that you will speak to your conclusion or what you learned in brief.] Introduction [What you will put here: In this introduction, some background is provided for the reader (sources are noted in the reference section at the end of the report).Typically, the introduction states the problem to be solved or the experiment to be performed and explains its purpose and significance. It also provides whatever background theory, previous research, or formulas the reader needs to understand to perform the experiment (or solve the problem).] Methods and Observations Materials Two Styrofoam coffee cups Thermometer Vinegar Mass Scale Measuring tablespoon and ½ teaspoon Lye Safety goggles Moore, Timothy MEASURING THE LYE: To begin the lab, I had to measure out a very small amount of lye. Because my scale is not precise at measuring small masses, I had to first calibrate my tablespoon to measure the mass of the lye that I needed for the lab. To do this, I [explain the process here]. I used ½ teaspoon of lye, calculating its mass as [enter the number of grams here]. THE CALORIMETER: I nested two Styrofoam coffee cups one inside of another to make a calorimeter. I then poured 100.0 mL of vinegar into the calorimeter. Next, I placed a thermometer in the vinegar and did not disturb it for three minutes. After that time, I recorded the temperature of the vinegar as [show the temperature here]. This was the initial temperature in the lab. I then added ½ teaspoon of lye to the vinegar, and began to stir the mixture with my thermometer. I began checking my thermometer about every 30 seconds, and I noticed that the temperature began to rise. Once the temperature was at a constant for two consecutive readings, I recorded the final temperature as [show the temperature here]. CALCULATIONS: I needed to use the equation q = mcΔT to calculate the results of the calorimetry experiment. I was given that the specific heat (c) of vinegar is 4.1 J/g. oC. I had to calculate the mass of the object (m) by multiplying the volume of the vinegar by its density and then adding the mass of the teaspoon of lye. I subtracted the initial temperature of the vinegar from the final temperature of the vinegar and lye mixture to get change in temperature (ΔT). I used all of this information to find the Joules of heat that was absorbed (q). [show your calculations here] In order to calculate the change in enthalpy (ΔT), I first converted grams of lye (NaOH) to moles of lye (NaOH), then divided the Joules of heat absorbed (q) by the number of moles of lye (NaOH). [show your calculations here] Conclusion My observations [You must explain, analyze, and interpret your results, being especially careful to explain any errors or problems. This is probably the single most important part of the report, since it Moore, Timothy is here that you demonstrate that you understand and can interpret what you have done. Read Dr. Wile’s explanation of the experiment in the text. Just don’t copy it word for word. ] References [Use MLA citation standards. This is commonly used in lab reports, but APA is used in some colleges too. I encourage you to do some of your own research too and add to the introduction section of the lab report from what you learn.