Counting Atoms
advertisement

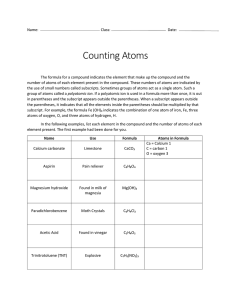
Counting Atoms Name ____________________________________________ The formula for a compound indicates the elements that make up the compound and the number of atoms of each element in the compound. These numbers of atoms are indicated by the use of small numbers, called subscripts. Sometimes groups of atoms act as a single atom—these are called polyatomic ions. If a polyatomic ion is used in the formula more than once, it is put in parentheses and the subscripts appear outside the parentheses. When a subscript appears outside the parentheses, it indicates that ALL the elements inside the parentheses should be multiplied by that subscript. For example, the formula Fe(OH)3 indicates the combination of one atom of iron (Fe), three atoms of oxygen (O) and three atoms of hydrogen (H). In the following examples, list each element in the compound and the number of atoms of each element present in the compound. If a coefficient is placed in front of a formula, multiply every element by that number. Name Calcium carbonate Aspirin Use Limestone Pain reliever Name Use Calcium dihydrogen phosphate Fertilizer Cellulose Found in wood products Aluminum hydroxide Used to make other aluminum compounds C9H8O4 Lanthanum carbonate Kidney medicine Formula 2 CaCO3 Atoms in Formula Ca = Calcium = 2 C = Carbon = 2 O = Oxygen = 6 Magnesium hydroxide Found in milk of magnesia Mg(OH)2 Ammonium sulfate Fertilizer Paradichlorobenzene Moth crystals 4 C6H4Cl2 Ferric nitrate (or iron nitrate) Used in etching metals Found in vinegar 2 C2H4O2 Cacodylic acid Herbicide Acetic acid Trinitrotoluene (TNT) Explosive C7H5(NO2)3 Formula 2 Ca(H2PO4)2 3 C6H7O2(OH)3 6 Al(OH)3 3 La2(CO3)3 4 (NH4)2SO4 5 Fe(NO3)3 2 (CH3)2AsO2H Atoms in Formula