Count the atoms and tell whether the chemical equation is balanced
advertisement

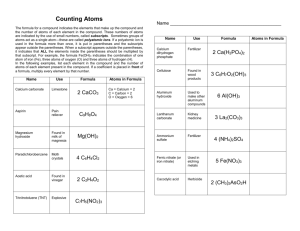
What is a chemical equation? Name _______________ When a chemical reaction occurs, it can be described by an equation. This shows the chemicals that react (called the reactants) on the left-hand side, and the chemicals that they produce (called the products) on the right-hand side. The chemicals can be represented by their names or by their chemical symbols. Unlike mathematical equations, the two sides are separated by an arrow, that indicates that the reactants form the products and not the other way round. Take a look at this chemical word equation: Aluminium + Oxygen Aluminium Oxide This is the equation for the burning of aluminium in oxygen. If we convert each of the chemical names into the appropriate symbols, we get the following: 2Al + O2 Al2O3 Practice Count the atoms and tell whether the chemical equation is balanced. Place a circle around the reactants and a rectangle around the products. 1. Zn + 2HCl ---> ZnCl2 + H2 ________________________ 2. S8 + F2 ---> SF6 ________________________ 3. 2C2H6 + 7O2 ---> 4CO2 + 6H2O ________________________ 4. C3H8 + 5O2 ---> 4H2O + 3CO2 ________________________ 5. C5H12 + 8O2 ---> 5CO2 + 6H2O ________________________ Counting Atoms Name _____________________ The formula for a compound indicates the elements that make up the compound and the number of atoms of each element present in the compound. These numbers of atoms are indicated by the use of small numbers called subscripts. When a subscript appears outside the parentheses, it indicates that all the elements inside the parentheses should be multiplied by that subscript. For example, the formula Fe(OH)3 indicates the combination of one atom of iron, Fe. three atoms of oxygen, O, and three atoms of hydrogen, H. In the following examples, list each element in the compound and the number of atoms of each element present. The first example has been done for you. You may already be familiar with some of the compounds. Name Use Formula Calcium carbonate limestone CaCO3 Aspirin pain reliever C9H8O4 Acetic Acid found in vinegar C2H402 Pyrite Fool’s Gold FeS2 Trinitrotoluene (TNT) explosive C7H5(NO2)3 Paradichlorobenzene moth balls C6H4Cl2 Atoms in Formula Ca = calcium – 1 C = carbon – 1 O = oxygen - 3