DOFETILIDE -- Tikosyn® 2000a Pfizer, Cardiovascular (Tee'-ko
advertisement

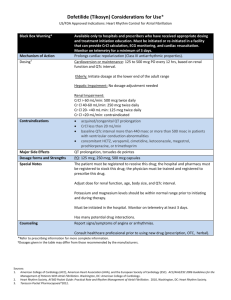
DOFETILIDE -- Tikosyn® 2000a Pfizer, Cardiovascular (Tee'-ko-syn) Avail: Available only to hospitals and physicians who have received dosing & tx education from Pfizer. 125, 250, 500 mcg capsules. Description: Oral anitarrhythmic used to convert Afib to NSR. Strong potential to induce ventricular arrhythmias. Chemically, the non-beta-blocking moiety of sotalol Mechanism of Action: Class III antiarrhythmic. Prolongs action potential duration, due to delayed repolarization. A selective potassiumchannel blocker. Other Class III agents: sotalol, amiodarone, ibutilide, bretylium. No effect on sodium (Class I), adrenergic alpha or beta receptors. No effect on BP and minor effect on HR. Dose: Patients initiated or reinitiated on dofetilide should be placed in a facility that provides CrCl calc, EKG monitoring, & cardiac resuscitation for a minimum of 3 days. Dose of dofetilide must be individualized based on calculated CrCl (Cockroft-Gault) and QTc interval. Dosage adjustment is critically important because QT interval prolongation and risk of ventricular arrhythmias are related to plasma concs. Dosing Algorithm 1. EKG assessment: Measure QTc. If QTc > 0.44 sec, do not use. 2. Starting dose: CrCl Starting Dose > 60 500 mcg po BID 40-60 250 mcg po BID 20-39 125 mcg po BID < 20 Contraindicated 2. Measure the QTc 2-3 hr after the first dose. If the QTc increased by >15% from baseline OR if the QTc is > 0.5 sec, subsequent doses should be reduced by 50% according to the package insert guidelines. 3. If QTc > 0.5 sec after the second dose, DC dofetilide. 4. Physicians may choose doses lower than the algorithm; however, any increases in dose requires rehospitalization. Indication: 1. Conversion of Afib/flutter to NSR 2. Maintenance of normal sinus rhythm (NSR) in pts w/ Afib/flutter of > 1 wk duration. Should be reserved or pts in whom Afib/flutter is highly symptomatic Adverse Reactions: 1. Ventricular arrhythmias: primarily Torsade de Pointes 0.8%, VTach assoc w/ QT interval prolongation (directly related to dofetilide plasma concs). 2. Other: headache 11%, chest pain 9%, dizziness 8% (all rates similar to placebo). Kinetics: Bioavailability > 90%. Half-life 10 hr. Primarily renally excreted (80%) as unchanged drug. Some active tubular secretion. Drug Interactions: 1. Inhibitors of cationic renal excretion: cimetidine, trimethoprim, ketoconazole. 2. Drugs that prolong QT interval: phenothiazine, bepridil, tricyclics, certain oral macrolides. 3. Class I or III antiarrhythmics: withhold for at least 3 half-lives. Amiodarone should be withdrawn for at least 3 months. Contraindications: 1. Congenital or acquired long QT syndromes. Pts with baseline QTc >440 msec. 2. Severe renal impairment (CrCl <20 ml/min). 3. Verapamil, cimetidine, trimethoprim, itraconazole, ketoconazole, prochlorperazine, megestrol. Itraconazole strongly inhibits CYP 3A4 -> increased dofetilide concs. Patient Info: 1. Take with or without food. 2. Take it at the same time each day. 3. Remind pt of drug interactions and the need for periodic monitoring of QTc and renal function. 4. If a dose is missed, do not double the next dose, but take the next dose at its usual time. 5. Notify MD if having abnormal fast heartbeats w/ dizziness or fainting. Other: Tikosyn hotline: 1-877-845-6796 (TIKOSYN) © 2000 New Drug Pocket Briefs