Density of Rubber Experiment 07
advertisement

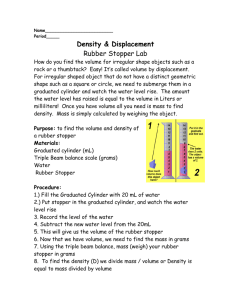
Density of Rubber Experiment Name ______________________ Period: 1 2 3 4 5 6 7 8 Purpose: To determine the density of rubber. Materials: 5 rubber stoppers #00, 0, 1, 2, and 3; digital balance; 100 ml graduated cylinder Procedure: 1. Weigh the #00 rubber stopper. Record the mass in your data table. 2. Add 50.0 ml of water to your 100 ml graduated cylinder. (Significant figures are VERY important!) Be sure to read the cylinder at eye level and at the bottom of the meniscus. 3. Slide the #00 stopper down the side of the graduated cylinder to avoid splashing and read the volume of water in the cylinder. Subtract the original volume of water from the new volume and record the result in your data table as the volume of the #00 stopper. (Remember, the volume of the stopper is only equal to the volume of displaced water.) 4. REPEAT steps #1-3 for EACH INDIVIDUAL remaining stopper, recording all data in your table. 5. Return the stoppers to the proper containers. Put all laboratory equipment AWAY, clean, but not necessarily dry! Graphing: 1. Prepare a graph of mass vs. volume where mass is the dependent variable and volume is the independent variable. 2. Label each axis with both the general term for the variable and in parenthesis, the unit for the variable. 3. Scale the axis so that is easy to plot points and at least ¾ of the sheet of graph paper is used. 4. Locate each data point by a small dot. Draw a small circle around each dot to indicate experimental error. 5. Draw a smooth curve or straight line that represents the pattern the points seem to follow. 6. DO NOT connect the dots with a series of straight lines! 7. Title your graph appropriately. Questions: 1. What were the variables in this experiment? 2. Is the graph showing a direct or indirect relationship between the two variables? 3. Create a mathematical equation that properly represents the relationship between the x values and the y values. 4. Determine the slope of the line. Be sure to use TWO points that are on the line. These may not necessarily be data points. Include the appropriate units for the slope. 5. Determine the slope of the line again. Be sure to use TWO different points that are on the line. These may not necessarily be data points. Include the appropriate units for the slope. 6. Compare your results for questions 5 and 6. Are they close? Should they be close? Explain your answers. 7. Take the equation that you created in #3 and properly substitute the labels (mass, volume, density) for the variables. 8. How is the density of rubber related to the graph? 9. What is the density of rubber? 10. Using the graph, determine the mass of 10.0 ml of rubber. 11. Using the graph, determine the volume of 15.0 grams of rubber. 12. From the given graph (not your experimental graph), create a data table for the information presented by the graph. Your data table should contain at least four data points for each substance.