Biochemistry and Physiology of muscle contraction
advertisement

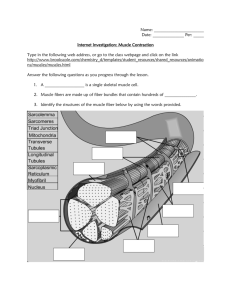
Biochemistry and Physiology of muscle contraction 1. Briefly outline the molecular basis of movement, identifying the motor molecules and principal control signals mediating axonal transport and other intracellular movement, cellular locomotion, and movement of the whole body by contraction of skeletal muscle. Skeletal muscle fibres rock! They are huge, multinuclear, bright red, and all end up in rigor mortis. Their structure is as follows: The sarcolemma is the plasma membrane of a muscle fibre, and surrounds the sarcoplasm. Like other plasma membranes, the sarcolemma has a characteristic transmembrane potential due to the unequal distribution of positive and negative charges across the membrane. In a skeletal muscle fiber, a sudden change in the transmembrane potential is the first step that leads to a contraction. Even though a skeletal muscle fiber is very large, all regions of the cell must contract simultaneously. Thus, the signal to contract must be distributed quickly throughout the interior of the cell. This signal is conducted through the transverse tubules (T tubules). T tubules form passageways through the muscle fiber, like a network of tunnels through a mountain. The T tubules have the same general properties as the sarcolemma, so electrical impulses conducted by the sarcolemma travel along the T tubules into the cell interior. These impulses, or action potentials, are the triggers for muscle fiber contraction. QuickTime™ and a decompressor are needed to see this picture. Inside the muscle fiber, branches of the T tubules encircle cylindrical structures called myofibrils. These consist of bundles of protein filaments called myofilaments. Thin filaments are composed primarily of actin, whereas thick filaments are composed primarily of myosin. A single thin filament contains four proteins: F-actin (aggregated G-actin), nebulin, tropomyosin, and troponin. A tropomyosin molecule is a double-stranded protein that covers active sites of G-actin. It is bound to one molecule of troponin midway along its length. Troponin is a molecule that consists of three globular subunits. The 1st subunit binds to tropomyosin the 2nd binds to G-actin to hold the Troponin-tropomyosin complex in position. The last subunit binds Ca2+ and causes a conformational change that exposes the actin binding sites. A thick filament contains roughly 300 myosin molecules, each made up of a pair of myosin subunits twisted around one another. The head has two globular protein subunits. When the myosin heads interact with thin filaments during a contraction, they are known as cross-bridges (see Muscle Contraction below). At each end of the skeletal muscle fiber, the myofibrils are anchored to the inner surface of the sarcolemma. In turn, the outer surface of the sarcolemma is attached to collagen fibers of the tendon or aponeurosis of the skeletal muscle. A muscle contraction begins when stored calcium ions (see next LO) are released into the sarcoplasm. These ions then diffuse into individual contractile units called sarcomeres. The sarcomere is the functional unit of skeletal muscle - the smallest component of a muscle fibre that can contract. A myofibril consists of approximately 10,000 sarcomeres. Interactions between the thick (dark band) and thin (light band) filaments of sarcomeres are responsible for muscle contraction. Differences in the size, density and distribution of thick and thin filaments give skeletal muscle its striated appearance. The A and I bands are formed by alternate stacked sets of thick and thin filaments that slightly overlap each other. The H zone is the area within the middle of the A band where the thin filaments do not reach. The I band consists of the remaining portion of thin filaments that do not project into the A band. In the middle of each I band is a dense, vertical Z line. When a skeletal muscle fiber contracts, (1) the H bands and I bands get smaller, (2) the zones of overlap get larger, (3) the Z lines move closer together, and (4) the width of the A band remains constant – all only if the thin filaments slide towards the centre of the sarcomere. QuickTime™ and a decompressor are needed to see this picture. QuickTime™ and a decompressor are needed to see this picture. 2. Outline the innervation of muscle and the events which lead to contraction following an action potential at the neuromuscular junction: - motor units, ion current at the neuromuscular junction, the role of calcium in triggering contraction Neuromuscular junction (motor end plate) The nervous system controls skeletal muscle contractions via impulses from motor neurons. The motor nerve and the group of muscle fibres it supplies is the motor unit. An axon meeting a muscle fibre forms a number of fine branches, and may innervate 100’s of muscle fibres. Each branch ends at a synaptic terminal, and interfaces at a junction (called the synaptic cleft) with the muscle fibre. The cytoplasm of the synaptic terminal contains mitochondria and vesicles filled with molecules of acetylcholine (neurotransmitter). the release of ACh from the synaptic terminal can alter the permeability of the sarcolemma and trigger the contraction of the muscle fiber. The initiation of a muscle contraction is summarised in the following steps. It is important to note that muscle fibres and excitable like nerve fibres, and respond to changes in resting potential in a similar way to nerves: Step 1 The Arrival of an Action Potential. The arrival of an electrical impulse, or action potential, at the synaptic terminal stimulates calcium channels to open so that Ca2+ ions enter the terminal from the ECF. Step 2 The Release of ACh. This permeability change to the membrane triggers the exocytosis of Acetylcholine (Ach) into the synaptic cleft. This is accomplished when vesicles in the synaptic terminal fuse with the plasma membrane of the neuron. Step 3 ACh Binding at the Motor End Plate. ACh molecules diffuse across the synaptic cleft and bind to ACh receptors on the surface of the sarcolemma at the motor end plate. ACh binding changes the permeability of the motor end plate to sodium ions, and sodium ions rush into the sarcoplasm. Step 4 Appearance of an Action Potential in the Sarcolemma. The sudden inrush of sodium ions results in the generation of an action potential in the sarcolemma. This impulse sweeps across the entire membrane surface, and travels inward along each T tubule. Step 5 Return to Initial State. Even before the action potential has spread across the entire sarcolemma, the ACh has been broken down by cholinesterase. This sequence of events can now be repeated should another action potential arrive at the synaptic terminal. The next phase, describing actual contraction, references the proteins of the sarcomere described above. Note - skeletal muscle fibers remove calcium ions from the sarcoplasm by actively transporting them into the ‘terminal cisternae’ of the sarcoplasmic reticulum (vs most cells which pump the calcium ions across their plasma membranes and into the ECF). The contraction cycle: Essentially cross bridges form between actin and myosin when thin actin filaments slide over thicker myosin filaments, and the muscle shortens. The process requires calcium, sodium, potassium, glucose and ATP. Step 1 Exposure of Active Sites. The calcium ions entering the sarcoplasm bind to troponin on Gactin. This binding weakens the bond between the troponin–tropomyosin complex and actin. The troponin molecule then changes position, rolling the tropomyosin molecule away from the active sites on actin and allowing interaction with the energised myosin heads. QuickTime™ and a decompressor are needed to see this picture. (a) In a resting sarcomere, the tropomyosin strands cover the active sites on the thin filaments, preventing cross-bridge formation. (b) When calcium ions enter the sarcomere, they bind to troponin, which rotates and swings the tropomyosin away from the active sites. (c) Crossbridge formation then occurs, and the contraction cycle begins. Step 2 Formation of Cross-Bridges. When the active sites are exposed, the energized myosin heads bind to them, forming cross-bridges. Step 3 Power Stroke. The myosin head pivots, bends and assumes its low-energy shape, which pulls on the actin filament, and ADP and its phosphate group are released. Step 4 Detachment of Cross-Bridges. When another ATP binds to the myosin head, the link between the active site on the actin molecule and the myosin head is broken. The active site is now exposed and able to form another cross-bridge. Step 5 Reactivation of Myosin. Myosin is returned to its high energy state before the next contraction, a process that uses ATP. The entire cycle will now be repeated, several times each second, as long as calcium ion concentrations remain elevated and ATP reserves are sufficient. Each power stroke shortens the sarcomere by about 0.5 percent, and because all the sarcomeres contract together, the entire muscle shortens at the same rate. QuickTime™ and a decompressor are needed to see this picture.