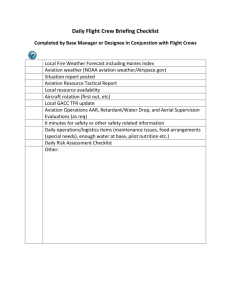
NPD - Institutional Repositories
advertisement

Copyright by Nicole Powell-Dunford 2008 The Capstone Committee for Nicole C. Powell-Dunford Certifies that this is the approved version of the following dissertation capstone: DYSMENORRHEA: An Aeromedical Clinical Practice Guideline Committee Robert Johnson, MD, MPH, MBA, Supervisor Richard Jennings, MD, MS James Vanderploeg, MD Janice Smith, MD __________________ Dean, Graduate School DYSMENORRHEA: An Aeromedical Clinical Practice Guideline by Nicole C. Powell-Dunford, MD Capstone Presented to the Faculty of the Graduate School of The University of Texas Medical Branch in Partial Fulfillment of the Requirements for the Degree of Master of Public Health The University of Texas Medical Branch August, 2008 Dedication This Capstone is dedicated to my brothers and sisters in Army Aviation Acknowledgements I would like to give sincere thanks to all the UTMB and Army RAM faculty for their support in this research, particularly Dr. Johnson, Dr. Jennings, Colonel Boneta and Colonel Albano. I am also especially grateful for the support of my husband Mike and my children Julia and Joshua. DYSMENORRHEA: An Aeromedical Clinical Practice Guideline Publication No._____________ Nicole Powell-Dunford, MPH The University of Texas Medical Branch, 2008 Supervisor: Robert Johnson This capstone proposes a clinical practice guideline on the management of dysmenorrhea, with attention to the occupational aspects of disease and treatment within the aviation community. This evidence based clinical practice guideline was developed through a systematic review of the literature with consideration of the physiological and operational aspects of the flight environment. Dysmenorrhea can have a significant impact on United States civil and military flight crews. A standardized aeromedical guideline can benefit flight crew members as well as the aeromedical community who cares for them. Table of Contents CHAPTER 1: INTRODUCTION A. Historical Context .....................................................................................1 B. Aeromedical Concerns .............................................................................2 C. Background and Literature Review..........................................................2 CHAPTER 2: DYSMENORREA .........................................................................4 A. Impact of Disease ......................................................................................4 B. Etiology…….............................................................................................4 C. Clinical Presentation & Diagnosis……………………………………….5 D. Epidemiologic Principles ..........................................................................5 E. Evidence Based Medicine & Strength of Recommendation Theory……..5 F. Clinical Practice Guidelines……………………………………………...7 CHAPTER 3: TREATMENT A. NSAIDS…………………………………………………………………..9 B OCPs……………………………………………………………………....9 C. Other Hormonal Treatments……………………………………………....9 Danazol………………………………………………………………..10 Depo Medroxy-Progesterone Acetate (Depo-Provera)……………....10 Gonadotropin Releasing Hormone Agonists……………………….....10 Levonorgestrel Releasing Intrauterine System………………………..11 Novel Hormonal Therapies…………………………………………...11 D. Surgery…………………………………………………………………....12 E. Non Traditional Therapies……………………………………………….12 Acupuncture/TENS………………………………………………….12 Lifestyle Modification……………………………………………….12 Fish Oil………………………………………………………………13 Herbs………………………………………………………………...13 Spinal Manipulation…………………………………………………13 Topical Heat Application……………………………………………13 Vasoactive Medications…………………………………………….14 Vitamins/Supplements……………………………………………...14 Magnesium…………………………………………………..14 Thiamine……………………………………………………..14 Vitamin E…………………………………………………….14 Zinc………………………………………………………….14 CHAPTER 4: AEROMEDICAL CONCERNS OF TREATMENT……………………………………………………………………16 A. NSAIDS……………………………………………………………….....16 B OCPs……………………………………………………………………...16 C. Other Hormonal Treatments……………………………………………...18 Danazol………………………………………………………………18 Depo Medroxy-Progesterone Acetate (Depo-Provera)……………..18 Gonadotropin Releasing Hormone Agonists………………………...19 Levonorgestrel Releasing Intrauterine System……………………....19 Novel Hormonal Therapies…………………………………………..19 D. Surgery……………………………………………………………………19 E. Non Traditional Therapies………………………………………………...20 Acupuncture/TENS………………………………………………...…20 Lifestyle Modification……………………………………………...…20 Fish Oil……………………………………………………………..…20 Herbs……………………………………………………………..…...20 Spinal Manipulation……………………………………………..……21 Topical Heat Application………………………………………..……21 Vasoactive Medications……………………………………….……..21 Vitamins/Supplements……………………………………………….21 Magnesium……………………………………………………21 Thiamine……………………………………………………...21 Vitamin E…………………………………………………..…22 Zinc……………………………………………………….….22 CHAPTER 5: DISCUSSION & CONCLUSION…………………………..…..23 A. Gap Analysis……………………………………………………….…….23 B. Study Types & Statistical Methods………………………………………23 C. Outcome……………………………………………………………….…23 Appendix A Aeromedical Clinical Practice Guideline…………………………...24 References…………………………………………………………………………27 Vita ………………………………………………………………………………..33 CHAPTER 1: INTRODUCTION Women have made remarkable progress in integration into the aviation community. Previously blocked from flight during the menstrual cycle due to unfounded beliefs that menstruation would contribute towards fatal mishaps, women now participate in every facet of modern aviation to include combat operations and international space station missions. Despite this, a shortage of aeromedical guidance exists with respect to a common gynecological condition in the civil aeromedical literature. Previous aeromedical guidance restricting flight during the normal physiologic condition of menstruation has been cast aside. Guidelines with respect to pilots with the common condition of dysmenorrhea are now proposed. Dysmenorrhea, painful menstruation that is sometimes associated with abnormal deposits of endometrial tissue in abnormal anatomical positions, may cause work limitations in a small percentage of women. Appropriate aeromedical management of this group is important. A. HISTORICAL CONTEXT Women have gained slow but steady progress in integration with aviation. Due to financial necessity and national defense, women entered aviation service in significant numbers during WWII, playing a critical support role as women Air Force service pilots. Yet the menstrual cycle was misconstrued as a safety of flight factor. In the 1935 Civil Aeronautics Handbook for Medical Examiners, the medical officer of the Aeronautics branch of the U.S. Dept of Commerce formally cautioned against flight during the menstrual cycle as well as 3 days prior and following the menstrual cycle due to an unsubstantiated link between menstruation and fatal accidents (Whitehead, 1935). The 1942 U.S. Army Fit to Fly handbook also cautioned against flight during the menstrual cycle due to the perception that accidents would ensue (Grow, 1942). Concerns about the menstrual cycle as a safety of flight factor were raised in the same decade. After interviewing many female endurance and racing pilots of the era, the assistant chief flight surgeon of the Connecticut Department of Aeronautics observed that the majority of women flew without significant symptoms during the menstrual cycle and that many noteworthy flight accomplishments occurred during the menstrual cycle (Holtz, 1941). In an analysis of U.S. Air Force training dropout and accident rates, the menstrual cycle was not found to be correlated with either (Monrerud, 1988). In fact, male pilots were found to have higher accidents rates when compared to female pilots (Lyons, 1992, Vail and Ekman, 1986). Although barred from combat flight under Title 10 U.S. Code 8549 of the Women’s Armed Services Integration Act of 1948 (Murnane, 2007), female pilots were finally authorized to perform combat flight duties on Congressional repeal of the Prohibition act in 1991. They have since not only served in combat, but as shuttle and space station commanders in civil aviation. Although outdated regulations in regards to menstrual cycle flight restrictions have been cast aside, little aeromedical guidance exists in modern regulations with regards to menstrual disorders, specifically dysmenorrhea. 1 B. AEROMEDICAL CONCERNS Prevalence of dysmenorrhea varies between 45 – 95%, making it the most common gynecological condition in the world (Proctor and Farquhar, 2006) with an estimated 10%-15% suffering from 1-3 days of incapacitating or work limiting symptoms every month (Ferri 2007, Dawood, 2006). This potentially translates to a significant number of the over 36,000 private and commercial women pilots today, 58 of which are captains on a single major U.S. carrier airline (American Airlines, 2007, FAA, 2007). Untreated severe dysmenorrhea can cause significant pain and loss of concentration, although likely contributing more to missed flight time ‘sick days’ than to sudden incapacitation due to gradual onset and predictable nature of the condition. Rarely associated with dysmenorrhea is the condition of spontaneous pneumothorax in areas of endometrial deposition, which is a special concern for crewmembers subject to altitude. Although first line medications are potentially compatible with flight duty, some second line therapies and herbal remedies are not compatible or are of unknown aeromedical safety. Lastly, aeromedical examiners may not be aware of newer therapies or guidelines for dysmenorrhea. A clinical guideline can serve to ensure aeromedically sound therapeutics, with appropriate flight restrictions when warranted. C. BACKGROUND & LITERATURE REVIEW The literature search for this capstone encompasses literature from peer-reviewed preventive medicine, aeromedical, gynecological and primary care sources as well as historical aeromedical publications. Other than references used for historical context and the limited information regarding non-traditional treatments, material previous to 2003 with respect to therapeutics was excluded from analysis in order to ascertain current medical knowledge of the past 5 years. Resources for literature review included the UTMB electronic medical library to include OVID, the Cochrane library, PubMed , and MdConsult. Additional data was found at the on-line AAFP (American Association of Family Physicians) and ACOG (American College of Obstetricians Gynecologists) medical repositories as well as the National Guideline Clearinghouse website which consolidates the clinical guidelines of major medical organizations. Aeromedical guidance was found at the AsMA (Aerospace Medical Association) and ASAM (American Society of Aerospace Medical Specialists) websites. The NTSB (National Transportation Safety Board) database was searched in order to determine incidence of medical condition involvement in aircraft accidents. Furthermore, the U.S. Department of Health and Human Services website was accessed for current U.S. preventative recommendations. Federal Aviation Administration (FAA) aeromedical clinical guidance was reviewed on-line at the official FAA website. Aeromedical Department of Defense (DoD) guidance was reviewed within the U.S. Army Aeromedical Resource Online (AERO, 2007) and the on-line U.S. Air Force aeromedical waiver guide, obtained through a secure Air Force knowledge exchange site. Search terms used included 2 ‘dysmenorrhea,’ ‘cyclic pelvic pain,’ as well as each of the individual names of therapies which will discussed for the treatment of dysmenorrhea. ‘Clot’ ‘DVT’, ‘deep venous thrombosis,’ PE’ and ‘pulmonary embolism’ were also used in an NTSB database search with regards to aviation mishaps related to complications from the treatments for dysmenorrhea. Data on traditional therapeutics prior to 2003 was excluded. 3 CHAPTER 2: DYSMENORRHEA A. IMPACT OF DISEASE Although no formal studies have analyzed the prevalence of dysmenorrhea in aviators, dysmenorrhea affects a significant percentage of the childbearing population in various degrees. Higher rates of dysmenorrhea are found in smokers, menorrhagic, nulliparous and younger women, with up to 15% experiencing work-limiting symptoms (Ferri 2007, Dawood, 2006). With close to 8,000 commercial female pilots today, this disease burden could translate to 7,000 pilots experiencing some degree of dysmenorrhea, with work implications for as many as 800 who experience incapacitating symptoms (FAA 2007). Due to gradual onset and predictable nature of symptoms of dysmenorrhea however, there have been no documented accidents or aviation mishaps from 1970 until present attributable to dysmenorrhea, with a lower overall mishap rate established for female pilots (NTSB, 2007, Lyons, 1992). B. ETIOLOGY Dysmenorrhea has been definitively linked with Prostaglandin F2α (PG F2) (Ferri, 2007), which causes uterine contractions and stimulates vasopressin release. Associated symptoms of severe dysmenorrhea, which may include nausea, vomiting, headaches, anxiety, fatigue, diarrhea, fainting, and abdominal bloating are mediated through prostaglandin (Ferri, 2007). Endometriosis, or abnormal deposition of uterine lining in extra-uterine locations, may also be associated with dysmenorrhea. Although asymptomatic subjects are just as likely to demonstrate endometrial deposits as women with symptomatic endometriosis on pathological specimen, extent of endometrial deposition correlates with symptom severity in symptomatic populations (French, 2005). Associated conditions, which have not been established as causal but consistently linked with dysmenorrhea, include smoking, depression/anxiety attempts to lose weight, heavy menstrual flow, nulliparity and social disruption (French, 2005). Whereas smoking could be proposed to lead to abnormal vascular changes that could contribute towards dysmenorrhea, psychosocial factors may affect pain perception and tolerance. Menorrhagia, or excessive menstrual bleeding in simple terms, is a predictor of dysmenorrhea and may exist independent of this condition (French, 2005). Whereas primary dysmenorrhea occurs in the setting of normal anatomy and physiology, secondary dysmenorrhea is cyclic menstrual pain from a pathological condition. Typical etiologies include endometriosis, adenomyosis, leiomyomas and, less commonly, chronic salpingitis, IUD use, congenital or acquired outflow tract obstruction, including cervical stenosis (Ferri, 2007). 4 C. CLINICAL PRESENTATION & DIAGNOSTICS Dysmenorrhea can vary from mild pain to severe incapacitation. Duration of symptoms varies between 1-3 days, with symptoms rarely exceeding 3 days. Iron deficient anemia, with risk for hypoxia-induced performance decrements, can result from menorrhagia. but is not linked independently with dysmenorrhea. Catamenial pneumothorax, occurring secondary to endometrial deposition, is an extremely rare condition and has never been linked with a mishap to date, but is a special aeromedical concern. Described in sporadic case reports and small case series (Hazelrigg, 2003, Mauer, 1958), this condition frequently manifests as sudden onset dyspnea, typically manifests right hemi-diaphram involvement and may require surgical treatment. Diagnosis of primary dysmenorrhea is typically straightforward with cramping pain occurring predictably at the time of menstruation in the setting of a normal physical examination. In most settings, no further diagnostic work up is required. Dysmenorrhea refractory to first line medications, with unusual history, abnormal physical exam or of longstanding nature is initially investigated through ultrasound, with surgical exploration reserved for severe cases and/or dysmenorrhea in the setting of abnormal diagnostic work- up. Ultrasonography is capable of visualizing cysts, fibroids and stage 3-4 endometriosis, with a 84% concordance rate with surgically staged endometriosis (Exacoustos et al., 2003). Saline infusion of the uterus or sonovagiograhy, is helpful in establishing rectovaginal endometriosis, frequently implicated in dysmenorrhea (Dessole et al., 2003). MRI has limited diagnostic utility, is more expensive and time intensive to obtain than ultrasonograhy and is not within the current standard of care (French, 2005, Stratton et al., 2003). D. EPIDEMIOLOGIC PRINCIPLES Dysmenorrhea is predominantly a disease of young women. Smokers and nulliparous women as well as women with depression and ongoing life stressors may benefit most from formalization of clinical practice guidelines due to increased prevalence in these populations. Aviators are a special epidemiological population with high levels of motivation and health consciousness. However as a group, they are prone to under-reporting. Strict treatment protocols that mandate grounding for any condition will decrease self-referral and treatment rates. Clinical guidelines including treatment options that permit continuation of flight status will likely increase diagnosis and treatment rates. E. EVIDENCE BASED MEDICINE & STRENGTH OF RECOMMENDATION THEORY With an expansion of medical literature, it has grown increasingly important to isolate clinically important and high quality medical data for diagnostic and treatment guidance. Malpractice coverage, Joint Commission on the Accreditation of Healthcare Organizations inspection and insurance reimbursement increasingly demand evidencebased medical practices. The Cochrane review database is explicitly dedicated to the 5 evaluation of the medical literature in order to establish the degree to which evidence supports specific medical practices. A number of family medicine and primary care organizations, including the American Association of Family Physicians (AAFP), have adopted formal stratification of clinical recommendations in the SORT or ‘Strength of recommendation taxonomy.’ system. Recommendations are rated with respect to quality, quantity, and consistency of evidence. ‘A’ level recommendations are based on ‘consistent, good quality, patientoriented evidence’, which would include high quality systematic reviews, randomized control trials (RCTs) and prospective cohort studies with good follow up. ‘B’ recommendations are based on ‘inconsistent or limited quality patient-oriented evidence,’ which includes lower-quality systematic reviews and RCTs, cohort studies with inconsistent results and/or poor follow-up, as well as case-control studies and prognostic case series. ‘C’ recommendations are based on ‘consensus, disease rather than patientoriented evidence, usual practice, opinion and/or non prognostic case series’ (AFP, 2007, Ebell et al., 2005). SORT class ‘B’ recommendations are further stratified as ‘probably effective’ and ’possibly effective’ whereas SORT class ‘C’ recommendations are further stratified into ‘uncertain effectiveness’ and ‘ineffective.’ The American College of Obstetric and Gynecology (ACOG ) uses a similar format for stratification of recommendations in which an ‘A’ recommendations are based on ‘good and consistent scientific evidence’. ‘B’ recommendations are based on ‘limited or inconsistent scientific evidence’ and ‘C’ recommendations are based primarily on ‘consensus and expert opinion’ (NGC/ACOG, 2004). Clinical Evidence Concise, a publication of the British Medical Journal, substantiates primary care medical interventions as ‘Beneficial’, ‘Likely to be beneficial,’ ‘Likely to be ineffective or harmful’ and ‘Unknown effectiveness.’ The U.S. Preventative Services Task Force (USPSTF, 2007) maintains another large repository of evidence-based recommendations, which are rated as ‘A’ through ‘D’ based on quality of evidence as well as magnitude of net benefit (benefit minus harms) with an ‘I’ designation for interventions that demonstrate insufficient evidence for application. Although the USPSTF currently has no formal recommendations for dysmenorrhea, the American College of Obstetric and Gynecology (ACOG) and American Association of Family Physicians (AAFP) have established guidelines for management of menstrual disorders which utilize ‘A’ through ‘C’ stratification systems. AGOC guidelines utilize an additional stratification system for ranking of evidence, which follows. I-Evidence obtained from at least one properly designed randomized controlled trial II-1 Evidence obtained from well-designed controlled trials without randomization II-2 Evidence obtained from well-designed cohort or case-control analytic studies, preferably from more than one center or research group II-3 Evidence obtained from multiple time series with or without the intervention or dramatic results in uncontrolled experiments. 6 III. Opinions of respected authorities, based on clinical experience, descriptive studies, or reports of expert committees (NGC/ACOG, 2004). Walkover comparison between SORT and other rating systems such as those of the British Medical Journal have been proposed. SORT ‘A’ recommendations are the equivalent of the Center for Evidence Based Medicine’s ‘consistent level I’ or the Clinical Evidence Concise and British Medical Journal’s ‘Beneficial’ ratings. SORT ‘C’ recommendations are equivalent to ‘Inconsistent’ or ‘Unknown Effectiveness’ interventions of these organizations respectively. SORT B recommendations are equivalent to ratings intermediate to these extremes (Ebell et al., 2004). Formal guidelines on management of dysmenorrhea have been established by the AAFP using the SORT criteria and guidelines on management of endometriosis have been established by the AGOC using both recommendation and quality of evidence stratification systems. Therefore, SORT recommendation levels for dysmenorrhea will be predominantly referenced in discussion of medical treatments in this paper. The ACOG’s stratification system will be referenced when pertinent as relating to the diagnosis of endometriosis. The National Guideline Clearinghouse (NGC) is “an initiative of the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services… originally (created) in partnership with the American Medical Association and the American Association of Health Plans (National Clearinghouse, 2007).” NGC is a databank of the clinical guidelines of major medical organizations, including over 250 guidelines currently in development. Guidelines for dysmenorrhea include a 2003 nursing guideline on management of cyclic pelvic pain as well as the 2004 ACOG guideline for management of endometriosis. F. CLINICAL PRACTICE GUIDELINES In order to streamline and summarize best evidence practices for the busy clinician, many professional societies such as the Aerospace Medical Association have embraced clinical practice guidelines, which formulate evidence-based recommendations. Guidelines differ from protocols in that they are more flexible and present multiple acceptable treatment options. Due to outcries against wide variation in medical practices throughout the 1970s, clinical practice guidelines evolved as a means of standardizing care (Wennberg and Gittelsohn, 1973). Standardized guidelines for treatment within a clinic, hospital or professional body have tremendous benefits. Medical providers are spared the task of analyzing conflicting data of unknown quality and can give clinical recommendations with confidence. Standardized clinical practice guidelines help to ensure that patients receive ideal management of a condition independent of variables such as ethnic origin, geographic location or insurer. On-line Clinical Practice Guidelines have the additional benefit of easy access, with capability of real-time updates as clinical developments occur. The American Society of Aeromedical Specialists, the U.S. Army and U.S. Air Force have on-line repositories and are models for this practice. U.S. Army has specific policy letter guidance on endometriosis (AERO, 2006, U.S. Army Aeromedical Policy Letters, 2006). 7 The U.S. Air Force has guidelines on both endometriosis as well as dysmenorrhea. Although the on-line American Society of Aeromedical Specialists clinical guideline repository includes a 2007 guideline on endometriosis, the Federal Aviation Administration (FAA) Guide for Aviation Medical Examiners deals with dysmenorrhea in a non- specific way, stating that “disorders such as sterility and menstrual irregularity are not usually of importance in qualification for medical certification” and that diagnostic evaluation is best left to a gynecologist (Guide for Aviation Medical Examiners, 2006). From a financial perspective, clinical guidelines prevent wastage of limited medical resources that may occur through application of ineffective therapies. Validation of clinical guidelines often occurs through peer review. For example ACOG’s final guidelines are evaluated by practicing obstetrician-gynecologists to include generalists and sub-specialists as well as the ACOG executive board (NGC/ACOG, 2004). 8 CHAPTER 3: TREATMENT A. NSAIDs Non-steroidal anti-inflammatories (NSAIDs) are a first line treatment for dysmenorrhea, a level A SORT recommendation (French, 2005). Importantly, NSAIDs not only alleviate pain but also inhibit prostaglandin. Although individual variation may exist, different NSAIDs are of similar or equal efficacy. A 2003 analysis of Cochrane Menstrual Disorders and Subfertility Group trials register determined that NSAIDs were significantly more effective for pain relief than placebo (OR 7.91, 95% CI 5.65 to 11.09), though overall adverse effects were also significantly more common (OR 1.52 95% CI 1.09 to 2.12) and that there was little evidence of the superiority of any individual NSAID with regard to either efficacy or safety (Marjoribanks et al., 2003). The NSAIDs studied in this analysis were high dose regimens and included ibuprofen (Motrin) 800mg three times daily, diclofenac (Voltaren) 50mg three times a day, mefenamic acid (Ponstel) 500mg loading dose followed by 250mg four times daily and naproxen (Naprosyn) 500-550mg twice daily. Patients taking lower doses, including typical over the counter doses, may not achieve effective symptom control (French, 2005). B. OCPs Oral contraceptive pills (OCPs) act as a treatment for dysmenorrhea through relative suppression of menstruation and can furthermore be prescribed for continuous use for complete menstrual suppression. Continuous use regimens are now available commercially; off-label use of continuous oral contraceptives for management of dysmenorrhea has been utilized for decades. A recent study with regards to continuous OCPs analyzed computerized databases (Cochrane Central Register of Controlled Trials, MEDLINE, EMBASE, POPLINE, LILACS) for trials using continuous or extended OCPs during the years 1966 to 2005. In the 6 studies reviewed, “The extended cycle group fared better in terms of headaches, genital irritation, tiredness, bloating, and menstrual pain. Five out of the six studies found that bleeding patterns were either equivalent between groups or improved with continuous-dosing regimens…. (with) similar contraceptive efficacy (i.e., pregnancy rates) and safety profiles (Edelman et al., 2005).” AAFP’s and ACOG’s most recent ratings of OCPs determine them to be a class ‘B’ intervention for dysmenorrhea and endometriosis respectively. AAFP further stratifies continuous OCP use to be ‘probably effective’ with standard oral and intravaginal OCP use as ‘possibly effective’ (French, 2005, NGC/ACOG, 2004). These classifications however must be taken in context with only limited data available for newer brands of extended cycle OCPs. C. OTHER HORMONAL TREATMENTS Approximately 10% of women with primary dysmenorrhea do not respond to treatment with either NSAIDs or oral contraceptives (Nasir and Bope, 2004, Proctor and Farquhar, 2006). Further hormonal treatments indicated for endometriosis-related 9 dysmenorrhea include danazol, depo-medroxyprogesterone acetate (Depo-Provera), Gonadotropin-releasing hormone agonists (GnRHAs) such as leuprolide acetate (Lupron) and the levonorgestrel releasing intrauterine system (Mirena), which have all received a SORT B ‘probably effective’ classification (French, 2005). New contraceptives such as the estrogen patch (Ortho Evra), injectable estrogen (Lunelle) and the vaginal ring (NuvaRing) also offer promise. These novel therapies do not appear in the Cochrane database as analyzed pools of clinical studies and have been assigned no formal SORT categories. Other hormonal based contraceptives demonstrate variable efficacy in the treatment of dysmenorrhea. Danazol Treatment of endometriosis-related dysmenorrhea with danazol for at least 6 months appears effective in most patients and is a class ‘A’ recommendation of ACOG (NGC/ACOG, 2004). Regardless of surgical confirmation, treatment of dysmenorrhea is rated as a SORT class ‘B’ ‘probably effective’ recommendation by the AAFP (French, 2005). Danazol in doses of 600 to 800 mg per day is as effective as 3 months of treatment with gonadotropin-releasing hormone agonsists (GnRHAs) for pain relief, but is associated with a significantly greater incidence of side effects (NGC/ACOG, 2004). Typical treatment is with 200—400 mg/day PO in two divided doses, with maximum 800mg/day for severe endometriosis and with therapy not to exceed 9 months, with 3-6 months typically required for benefit. The cost of treatment with danazol is about one third less than treatment with a GnRHA, but nearly twice as costly as treatment with oral contraceptives and oral depot medroxyprogesterone acetate (NGC/ACOG, 2004). Depo Medroxy-Progesterone Acetate (Depo-Provera) An intramuscular injection of 150mg depo medroxy-progesterone acetate (DepoProvera) once every three months is a convenient, off-label hormonal method of endometrial involution with a class ‘B’ ‘probably effective’ rating and class ‘B’ with ‘limited or inconsistent scientific evidence’ by AAFP and ACOG for the treatment of endometriosis and dysmenorrhea respectively. Associated with amenorrhea in over 75% of users after 1 year of use, it may be very efficacious in the treatment of dysmenorrhea for those taking this contraceptive. 104 mg Depo Sub-Q was subsequently specifically FDA approved for management of endometriosis, with the same bone density precautions as the 150mg IM Depo Provera injection (Gold Standard, 2007). Gonadotropin Releasing Hormone Agonists Treatment of biopsy proven endometriosis with the gonadotropin-releasing hormone agonists (GnRHAs), such as intramuscular leuprolide (Lupron), for at least 3 months is a class ‘A’ recommendation of the ACOG (NGC/ACOG, 2004). Treatment of chronic pelvic pain without surgical confirmation of endometriosis is a class ‘B’ 10 recommendation of ACOG while treatment of dysmenorrhea with lupron is an AAFP class ‘B’ ‘probably effective’ recommendation (French, 2005, NGC/ACOG, 2004). GnRHAs also include goserelin acetate (Zoladex), which is subcutaneous, and naferelin (Synarel), which is a nasal spray (Mahutte and Arici, 2003). All are associated with reduced bone density and vasomotor symptoms. However, when pain relief supports continued therapy, the addition of add-back therapy (progestins alone, bisphosphonates, progestins and estrogens, pulsatile parathyroid hormone and/or nasal calcitonin) reduces or eliminates GnRHA-induced bone mineral loss without reducing the efficacy of pain relief. Leuprolide (Lupron) is administered as an injection 3.75 mg IM once monthly or alternatively, 11.25 mg IM once every 3 months, for a maximum of 6 months. Either leuprolide dosage may be co-administered with norethindrone acetate (5 mg PO daily) (NGC/ACOG, 2004). Levonorgestrel Releasing Intrauterine System The levonorgestrel releasing intrauterine system (Mirena Intra-Uterine Device) releases levonorgestrel (20 µ/day) over the course of five years, causing up to 50% of women using it to achieve amenorrhea after 12 months. A reduction in dysmenorrhea, spontaneously reported in clinical trials, was also observed in a one-year random controlled trial of women with endometriosis (Proctor and Farquhar, 2006). The device has demonstrated good long-term acceptability (Baldaszti, 2003) and is a SORT class ‘B’ ‘possibly effective’ intervention for dysmenorrhea (French, 2005). Complications such as misplacement or infection requiring early removal are rare. Novel Hormonal Therapies The estrogen patch requires weekly application but is not currently FDA approved for dysmenorrhea. The patch is a small (2 cm2) adhesive square that releases 150 g of the progestin norelgestromin and 20 g of the estrogen ethinyl estradiol daily. Transdermal application once weekly may improve compliance and afford greater medication tolerability in some patients. Lunelle is a once-a-month injectable contraceptive containing 25 mg medroxyprogesterone acetate and 5 mg estradiol cypionate. It has not been formally studied in the treatment of dysmenorrhea. The vaginal ring (NuvaRing: etonogestrel and ethinyl estradiol) is a lightweight, small single-size contraceptive vaginal ring that releases 15 g of ethinyl estradiol and 120 g of etonogestrel daily; It is removed every three weeks for a single week. Although it is specifically cited as non-FDA approved for dysmenorrhea, it may afford a more convenient method of hormonal delivery for this syndrome. NuvaRing may need to be used for 6-9 months in order to be effective in the involution of endometrial deposits (Gold Standard, 2007). 11 D. SURGERY Surgical treatments for endometriosis-related dysmenorrheal include excision, endocoagulation, electrocautery, laser vaporization and hysterectomy with or without bilateral salpingoophrectomy as outlined in the ACOG clinical guideline on management of endometriosis (NGC/ACOG, 2004). Compared to laparotomy, operative laparoscopy for surgical treatment of pelvic pain related to endometriosis is associated with more rapid recovery, the potential to decrease postoperative adhesion formation, and lower complication rates (NGC/ACOG, 2004). The benefits of surgical interruption of the pelvic nerve pathways are still not fully developed and are considered a SORT class ‘C’ ‘uncertain effectiveness’ recommendation (French, 2005, Proctor et al., 2004). Hysterectomy is a SORT class ‘B’ ‘probably effective’ treatment recommendation of the AAFP (French, 2005). E. NON-TRADITIONAL THERAPIES Potentially beneficial non-traditional therapies that have undergone scientific review include acupuncture, behavioral modification, fish oil, herb and vitamin supplementation, as well as topical heat application, spinal manipulation and the use of off label vasoactive medications. Acupuncture/TENS (Transcutaneous Electric Nerve Stimulation) Both these treatment modalities were studied through a Cochrane systemic review and were found to be potentially beneficial in a number of small trials, resulting in a SORT ‘B’ ‘possibly effective’ treatment recommendation for acupuncture, acupressure and TENS (French, 2005, Proctor et al., 2002). Lifestyle Modification Although frequently recommended, exercise and behavioral intervention have not demonstrated clear effectiveness and are considered to be class ‘C’ SORT recommendations (French, 2005). Adherence to a low fat vegetarian diet, is a SORT class ‘B’ ‘possibly effective’ recommendation. A more recent 2007 Cochrane analysis of the medical literature reveal only mixed results for trials involving use of relaxation techniques (Proctor et al., 2007). Tobacco cessation, although not formally studied, may be a promising intervention. Responsible for the vast majority of all lung cancer, which is now the number one cause of cancer deaths in women, smoking has been established as a risk factor for dysmenorrhea. Although full cessation is difficult, even limited cessation around time of impending menses may offer benefit and is part of a recommended clinical guideline for nurse practitioners (NGC/Nurses Professional Association, 2003). 12 Fish Oil Fish oil supplementation has been determined to be a SORT class ‘B’ ‘possibly effective’ recommendation of the AAFP (French, 2005). A small RCT found that 2 g of fish oil (omega-3 polyunsaturated fatty acids) daily significantly reduced pain in 21 adolescent patients compared to 21 controls using the Cox Menstrual Symptom Scale with a mean change from 69.9 to 44.0 ( p < 0.0004) on this rating scale (Harel, 1996). High intake of marine n-3 fatty acids correlated with milder menstrual symptoms in 181 Danish women in an observational study (Deutch, 1995). Omacor, a fish oil pill with primary indication for hypertriglyceridemia, contains 4 mg (6 IU) of vitamin E as well as 1 gram of omega-3 fatty acids. Herbs Angelica (Dong Quai, Dang Gui, Tang Kuei) and Rose Tea (Toki-shakuyaku-san) are herbs that have been used in the treatment of dysmenorrhea. Angelica has been recommended for a multitude of generic gynecological complaints ranging from atrophic vaginitis to menopause to dysmenorrhea, but has not been rigorously studied for dysmenorrhea. The ACOG clinical guidelines do not cite angelica among the limited number of botanicals demonstrating even limited evidence of efficacy and furthermore strongly counsels against this therapy due to the non-regulated nature of botanical use (NGC/ACOG, 2006). Rose tea has been determined to be a class ‘B’ ‘possibly effective’ intervention by the AAFP (French, 2005). In 70 adolescents, rose tea was found to be efficacious compared to controls in management of dysmenorrhea at 1, 3 and 6 months (Taiwan, 2005). Spinal Manipulation Cochrane analysis has determined there to be insufficient evidence for recommendation of spinal manipulation in the treatment of dysmenorrhea. Four trials of high velocity low amplitude manipulation did not establish significant differences between sham and actual treatment; a small study involving the Toftness technique suggested some difference between sham treatment, but this study was found to be methologically flawed (Proctor et al. 2004). AAFP specifically recommends against this therapy, with a class ‘C’ ‘ineffective’ categorization (French, 2005). Topical Heat Application AAFP currently cites topical heat application as a SORT class ‘B’ ‘possibly effective’ treatment modality, which has few adverse effects (French, 2005, Proctor and Farquhar, 2002). 13 Vasoactive Medications Glyceryl trinitrate, nifedipine and terbutaline were briefly explored for the management of dysmenorrhea in the 1970s. Although demonstrating potential utility, these medications have never become established treatments; Nifedipine and terbutaline are referenced by AAFP as SORT class ‘C’ therapies of ‘uncertain effectiveness’ (French, 2005). Glycerine trinitrite has been studied more recently (Facchinetti et al., 2002) but had significant limitation in utility due to its association with headaches. Vitamins/Supplements Magnesium, Thiamine, Vitamin E and Zinc have been used in the treatment of dysmenorrhea. Magnesium Several small clinical trials, involving 20-50 women revealed that magnesium may be beneficial in reducing symptoms of dysmenorrhea. Magnesium was taken for a maximum of six months in various doses and regimens in these trials (Proctor et al., 2001). Magnesium salicylate is currently cited as an off- label treatment for dysmenorrhea with a recommended dosage of 600 mg every 4 hours with a maximum 4.8 g daily, with therapeutic effects through salicylate effects. It may serve as a useful adjuvant therapy to those unable to tolerate NSAIDs but has not been studied as part of major Cochrane analyses and has not been given a SORT rating. Thiamine Thiamine received an AAFP SORT rating of B, ‘possibly effective’, with positive systematic reviews and one study showing that 100 mg of thiamine (vitamin B-1) taken daily cured 87% of patients for up to two months after treatment (Proctor and Murphy, 2001). Vitamin E Treatment with 2500 IU of Vitamin E was proven useful in a single small trial. Daily use for 5 days beginning 2 days prior to menses may be effective in treatment of dysmenorrhea, a SORT ‘B’ ‘possibly effective’ intervention according to AAFP guidelines (French, 2005). Zinc A small case series has demonstrated zinc, “in 1–3 30-mg doses given daily for one to four days prior to onset of menses, prevents essentially all warning of menses and all menstrual cramping” (Eby, 2007). A previous study also revealed a positive impact of zinc on menstrual cramping (Eby et al., 1984). Menstrual cramping occurred at 15 mg but not 30mg of supplementation in patients treated with zinc (Goei, 1982) and effects were seen with zinc gluconate specifically. Lozenger delivery of zinc appeared to deliver quicker results (Prasad, 2000). Zinc oxide does not have physiologic utility and should 14 not be used. Although the mechanism by which zinc would improve menstrual cramping is not understood, relative deficiency may play a role. “The United States recommended daily allowance (RDA) for zinc has been 15 mg for many years, but it has recently been lowered to 8 mg per day for women, while the median intake of zinc from food in the United States has been found to be 9 mg per day for women…therapeutic doses of 30mg a daily for 3-5 days appear safe and do not interfere with copper metabolism (Eby, 2007).” Zinc supplementation has not received a SORT rating. 15 CHAPTER 4: AEROMEDICAL CONCERNS OF TREATMENT In this section, aeromedical concerns of first line medical therapies as well as those of non-traditional medical therapy for dysmenorrhea are discussed. In both military and civil aviation, aeromedical guidance about medications such as NSAIDs and hormonal therapies has been previously outlined. Non-traditional medication and supplements may have varying doses and are not regulated by the FDA (Food and Drug Administration). For this reason, all but 4 are explicitly grounding for use in U.S. Army aviation, none of which are indicated for dysmenorrhea (AERO, 2006). At the other extreme, little written guidance or specific prohibition exists in civil aviation medicine with regards to non-traditional therapies. Side effects and medication interaction potential in traditional as well as non-traditional treatments for dysmenorrhea are discussed here in an aeromedical context. A. NSAIDs Aeromedical concerns of NSAIDs include risk for gastric/duodenal bleeding and perforation, with potential for chronic anemia, incapacitating pain and/or acute blood loss. These risks are elevated in patients taking high dose regimens, and also increase with age. Overall risk of GI bleeding with high dose NSAIDs is low, with less than 1% of Motrin users experiencing ‘serious GI events’ which include ulcer with or without bleeding (Gold Standard, 2007). Anecdotally, acetaminophen has been selected over NSAIDs in combat aviation for minor menstrual pain when fully responsive to this medication due to lower risk for excessive trauma and/or forward surgical related bleeding. An on-line Aeromedical Examiner Guidebook reference cites upset GI tract, itching, dizziness and water retention as concerns for ibuprofen use (Guide for Aviation Medical Examiners tutorial, 2006). Caution must be used with high-dose NSAIDs, especially when combined with non-disclosed over the counter medications containing NSAIDs. Over the counter use of medication should be regulated in order to ensure high dose NSAIDs with differing brand names are not used simultaneously. Duration of use should be limited to just prior to symptom onset until normal day of resolution of symptoms. ASAMs cites NSAIDs as a waiverable class of medication in the management of endometriosis (ASAMS, 2007). The U.S. Army Policy Letters cites NSAIDs and analgesic equivalents as ‘not requiring a waiver’ (AERO, 2006). B. OCPs Aeromedical concerns for oral contraceptives include risk for DVT (deep venous thrombosis), which is four times higher in users of low-dose estrogen preparations containing older progesterone agents (levonorgestrel, lynoestrenol, and norethisterone) than the risk is for non users of OCPs (Hoffman, 2005). However, overall myocardial infarction and stroke risk is reduced in OCP users, likely because of estrogen's favorable influence on lipid profile (Hoffman, 2005). OCPs formulated with newer progestins 16 (desogestrel, gestodene, and norgestimate) are associated with a higher risk of DVT when compared to traditional OCPs (Hoffman, 2005). OCPs users who are pro-thrombotic face even higher than average risk with OCP use; carriers of Factor V Leiden deficiency mutation are at 35-fold increased risk. (Hoffman, 2005). Carriers of prothrombin gene (PT G20210A), which appears in 1-6% of Caucasian women but is uncommon in other ethnicities, have a 16-fold risk of DVT (Press et al., 2002). To present these risks in context, it is helpful to realize the overall small prevalence of a first time occurrence of a DVT – the incidence in the general population being 0.008 episodes per 100 person-years, with OCP users still having an overall low incidence of 0.03 episodes per 100 person-years and OCP users with the factor V Leiden mutation 0.285 episodes per 100 person-years (Hoffman, 2005). DVT has not been associated with any in-flight fatalities on a NTBS search dating from 1970 to present. OCPs are preventative against several medical conditions that present significant risk for in-flight incapacitation such as ovarian torsion, symptomatic hemorrhagic cyst and ruptured ectopic pregnancy. In terms of longitudinal health of the individual OCP user, it must also be remembered that pregnancy itself carries a much higher risk of PE/ DVT than OCPs. Pregnancy at some point will almost always temporarily preclude flight duties and may be the very least concern in the event of an unintended gestation. OCP users with hypertension may experience increase in blood pressure. Acne and headaches may worsen or improve in patients with either condition. New OCP users may also experience bloating, nausea and menstrual irregularities; these are frequently transient and resolve within 3 months of initial use. In terms of ocular risk, users of hormonal contraception experience slightly increased risk of optic neuritis, diplopia, loss of vision and retinal thrombosis with estrogens being specifically related to keratoconus. Pilots who begin new hormonal therapy should be advised to seek evaluation for any change in vision. Headaches and emotional changes may occur in some women, but significant gastrointestinal side effects are rare. Given potential for side effects that might have aeromedical implications, a brief period of initial flight restriction is appropriate in new OCP users in order to ensure lack of flight interference. U.S. Army policy letters conservatively advise at least 24 hours of grounding for the first time use of most medications (AERO, 2006). Intravaginal oral contraceptive application of 30mcg ethinyl estradiol and 150mg levonorgestrel may afford greater side effect tolerability as demonstrated in a RCT of 143 women (Ziaei, 2002) and may be a useful means of treating dysmenorrhea in women with persistent side effects from oral contraceptives. Oral contraceptives use for endometriosis is cited as waiverable in ASAMS clinical guidelines (ASAMS, 2007). Progestins, to include combination oral contraceptives, are cited as potentially waiverable in the U.S. Army Aeromedical Policy letters (AERO, 2006). 17 C. OTHER HORMONAL TREATMENTS Danazol Danazol is noteworthy for the induction of androgenic and anabolic side effects as well as a pseudomenopausal state. Diaphoresis, flushing, headache, nausea/vomiting, photosensitivity, pruritis and visual changes may occur, along with increased risk for stroke, thromboembolism, Guillain Barre syndrome and Stevens Johnson syndrome (Gold Standard, 2007). U.S. Army aviators who are prescribed this medication for endometriosis may be granted a waiver once symptoms have stabilized (AERO, 2006). Danazol is also cited as a waiver-requiring medication by the ASAMS (ASAMS, 2007). Refractory symptoms or side effects that interfere with flight mandate at least temporary grounding. Depo Medroxy-Progesterone Acetate (Depo-Provera) Although not associated with thrombo-embolic events that would immediately impact flight safety, Depo Provera may be associated with long-term risk for bone loss. In November 2004, Pfizer Pharmaceuticals added a black box warning that “women who use Depo-Provera Contraceptive Injection may lose significant bone mineral density. Bone loss is greater with increasing duration of use and may not be completely reversible…. (Depo-Provera) should be used as a long-term birth control method (e.g. longer than 2 years) only if other birth control methods are inadequate” (Nicoletti and Tonelli, 2005). In the setting of conflicting data on reversibility of bone demineralization, some clinicians discount demineralization as an insignificant risk of Depo-Provera use (Nicoletti and Tonelli, 2005). However the potential long-term risk is an important consideration for military pilots, who must maintain excellent fitness and weight bearing capacity, as well as astronauts who assume the additional bone reduction risk of microgravity. Additionally, transient breakthrough bleeding occurs in 38% to 47% of patients (Olive, 2007). Depo-Provera is listed as waiverable for the treatment of endometriosis by ASAMS and the U.S. Army Policy Letters (AERO, 2006, ASAMS, 2007). Gonadotropin Releasing Hormone Agonists With significant side effects of hot flushes, vaginal dryness, and insomnia being common, GnRHA use is frequently incompatible with flight. Moreover, a 5-6% decrease in bone mineral density has been established in GnRHA users, presenting readiness and long-term health issues to military pilots and astronauts. Utilization of add-back therapy can prevent bone loss for up to 1 year and may be helpful in side effect alleviation; GnRHA use of even short duration should be coupled with add-back therapy in this population (Mahutte and Arici, 2003, Olive, 2007). U.S. Army aviators who are prescribed this medication for endometriosis may be granted a waiver once symptoms 18 have stabilized (AERO, 2006). ASAMS also considers this medication to have potential for waiver action when used in the treatment of endometriosis (ASAMS, 2007). Levonorgestrel Releasing Intrauterine System Side effects of hormonal based IUDs include potentially aeromedically significant “dizziness, headaches, breast tenderness, nausea, vomiting (Shulman et al., 2004).” However risk for most of these side effects is relatively small, with 23% experiencing some nausea, 5% experiencing vomiting and approximately 11% experiencing other symptoms with initial use (Gold Standard, 2007). Like other progestins, this therapy is cited as potentially waiverable by in the U.S. Army Policy Letters (AERO, 2007). Novel Hormonal Therapies The estrogen patch, injectable estrogen and vaginal ring have unique aeromedical concerns. In the case of the estrogen patch, two case-control studies compared DVT rates between patch and OCP users using insurance and medical record data. Although one study found no difference in rates, the unpublished “Ingenix study” found a twofold increased risk of thromboembolism in patch vs. OCP users (odds ratio, 2.2; CI, 1.3–3.8) (Jick et al., 2006, Orthoevra.com, 2006, Swica, 2007). The Ingenix study has not been published in a peer-reviewed journal and many clinicians feel that the FDA black box warning which the Ingenix study generated is unwarranted (Swica, 2007). None the less, until true DVT risk is further established, pilots at risk for DVT through genetics or frequent long duration flight are not ideal candidates for the estrogen patch in the management of dysmenorrhea. Injectable estrogen (Lunelle) is a once-a-month injectable contraceptive containing 25 mg medroxyprogesterone acetate (MPA) and 5 mg estradiol cypionate (E2C) (MPA/E2C: Lunelle; Pfizer, Inc.) and presents similar aeromedical concerns with respect to estrogen effects. The Vaginal Ring (NuvaRing) (etonogestrel and ethinyl estradiol) is classified as low estrogen, and is cited as having similar risk profile to standard OCPs, with additional small risk of toxic shock like syndrome. Risk for dislodgement with flight has not been analyzed but rates of non-specific ‘device related events’, which include dyspareunia as well as dislodgement, are approximately 4% (Swica, 2007). The NuvaRing outer diameter is 5.4 cm with a cross-sectional diameter of 0.4 cm. Pilots with acrobatic, negative G and other highly demanding flight profiles should consider a trial of relatively level flight with initial use if fit is uncertain. D. SURGERY Following appropriate post-operative recovery and stabilized symptomatology, return to flight duties is appropriate. Surgical treatment of endometriosis in particular is fully endorsed as a waiverable condition in the U.S. Army Aeromedical policy letters (AERO, 2006). 19 E. NON-TRADITIONAL THERAPIES Acupuncture/TENS (Transcutaneous Electric Nerve Stimulation) Acupuncture, when controlling symptoms, may afford ideal pain management due to extremely low side effect profile. Although no FAA restrictions apply, acupuncture use must be reported on interval flight physicals in civil aviation. The Civil Aerospace Medical Institute stresses the importance of ensuring that the underlying condition is fully explored and reported (Mohler, 2002). Lifestyle Modification Tobacco cessation may be associated with irritability as well as increased rates of sore throat, cough, hearing difficulty, and mouth ulcers in the first 6 weeks of cessation, presenting potential distraction from flight (Ussher at al., 2003). However sustained tobacco cessation affords significant increases in overall lifetime survival and may reduce the self-imposed stress of hypoxia in pilots (Edwards, 2004). A well-balanced low fat vegetarian diet with adequate protein supplementation should pose no aeromedical concern; furthermore aviators who do not exceed BMI may have greater tolerance for physiologic stresses of flight. Although not clearly indicated for treatment of dysmenorrhea, exercise affords multiple health benefits. Weight training rather than excessive jogging is traditionally preferred for pilots subject to high G environments secondary to G-lock considerations (Burton and Whinnery, 2002). Fish oil N-3 fatty acids (FAs) from fish or fish-oil supplements such as Omacor have demonstrated ability to reduce rates of cardiac, sudden and all-cause mortality with further potential for stroke reduction and little adverse risk (Wang et al., 2006). Omacor’s only major adverse effect is burping and fishy taste (Bays, 2006), which would not preclude flight duties unless burping is distracting from flight. Herbs The use of angelica (Dong Quai, Dang Gui, Tang Kuei) and rose tea (Tokishakuyaku-san) have aeromedical sifnificance. The FAA advises that aeromedical examiners (AMEs) use caution with use of angelica during pregnancy and breast-feeding (Guide for Medical Examiners tutorial. 2006). No other non-traditional treatment of dysmenorrhea is discussed in the AME Guidebook, although guidance is given to the AME to evaluate the underlying condition and to caution aviators against the ‘more is better’ approach to self-medication. Herbal products are notorious for inconsistent labeling of concentrations and non- disclosure of full contents. Without reliability in deliverance, presence of evidence based research and safety regulations, herbal products cannot be recommended for aeromedical use. Aside from a limited number of products, which can be only used under the supervision of a flight surgeon, the U.S. Army Aviation Policy Letters categorizes virtually all herbal supplements as grounding (AERO, 2006). 20 Spinal manipulation Although no adverse events compared to sham therapy were determined in small studies involving treatment of dysmenorrhea, a lack of effectiveness of this therapy along with the potential for iatrogenic musculoskeletal disturbance exists. Therefore spinal manipulation cannot be recommended for aviators for dysmenorrhea. Topical Heat Application Although potentially useful in symptom alleviation, undertaking flight with reliance on this method to resolve distracting symptoms is problematic. Application of heat during flight may be burdensome and less effective with temperature declinations associated with flight. Vasoactive Medications Glyceryl trinitrate ,nifedipine and terbutaline have completely unacceptable risk profiles for flight given sympathomimetic and vasodilatory actions (Gold Standard, 2007), leading to high risk for incapacitation. Vitamins/Supplements Magnesium Magnesium salicylate’s major limitations are GI side effects, which may affect up to 30% of individuals (Gold Standard, 2007). Long term and high dose use of salicylates are also associated with tinnitus and hearing loss, important aeromedical considerations. Magnesium oxide and magnesium sulfate both have laxative effects that could be problematic for flight. Ensuring daily intake of 310-320 mg of magnesium, the recommended daily allowances of magnesium, may be prudent in women with dysmenorrhea. However additional supplementation beyond this amount may pose aeromedical risk. Salicylate use in the combat aviation setting may confer unacceptable risk of bleeding in the event of trauma and/or forward surgical operative requirement. Thiamine Hypersensitivity involving thiamine is rare, however hyperthyroid like symptoms of irritability, restlessness, headache, tremor, palpitations, anorexia and nausea/vomiting can occur with chronic large oral doses of thiamine, resolving when supplementation is stopped (Gold Standard, 2007). Recommended daily allowance is only 1.1 mg daily, far below the 100 mg doses used for treatment of dysmenorrhea and insufficient data exists on upper limits permissible in healthy subjects. Thiamine supplementation with 100 mg daily for dysmenorrhea appeared to be safe in studies involving 500 East Indian women 21 of unknown baseline serum thiamine levels (Proctor and Furquhar, 2002), but high dose supplementation should be studied further before being considered a safe therapy for general aeromedical use. Vitamin E Vitamin E and all fat-soluble vitamins are not considered appropriate for flight under army aeromedical policy due to potential for long term over accumulation. Prolonged use of large doses of vitamin E (400-800 Units daily) has caused nausea/vomiting; diarrhea; breast enlargement; fatigue; intestinal cramps; weakness; blurred vision; headache; and rash. Higher doses exceeding 800 Units per day can impact thyroid, pituitary, and adrenal hormone metabolism as well as bleeding time (Gold Standard, 2007). Lower doses, such as those included in Omacor, are safe for use in aviation. The 5-day treatment with 2,500 IU of Vitamin E, although effective in symptom reduction, may allow potential for side effects with long-term use and cannot be recommended for individuals on a flight status. Zinc Zinc supplementation should not exceed the upper tolerable limits of 40mg/day for adults (Gold Standard, 2004). The most common side effects with high dose zinc supplementation include nausea, abdominal pain and dyspepsia. Sideroblastic anemia may occur secondary to zinc induced copper deficiency (Gold Standard, 2007) and is a significant aeromedical concern with long-term use of high dose zinc supplementation. 22 CHAPTER 5: DISCUSSION & CONCLUSION A. GAP ANALYSIS Despite increasing participation of women in aviation, a gap exists in civilian aeromedical clinical guidelines for dysmenorrhea, a common condition. Military aeromedical guidelines, such as the Army aeromedical policy letters, contain guidelines for endometriosis but not primary dysmenorrhea. B. DATA & METHODS The clinical guideline was developed using literature search as previously discussed, in conjunction with senior aeromedical and obstetrical staff review. Aeromedical concerns were based upon typical side effects and potential adverse outcomes of therapies as delineated in 2007 Gold Standard pharmaceutical reference as well as those cited with previous DoD and civilian aeromedical references. This capstone has resulted in a clinical guideline for primary dysmenorrhea as a waiverable medical condition for flight when symptoms are controlled through appropriate therapeutics as outlined fully in capstone appendix. C. STUDY TYPES & STATISTICAL METHODS Data substantiating standard medical and surgical treatment recommendations were derived from randomized controlled trials. Guidance on non-traditional modalities as well as innovative new modalities was derived from existing knowledge from case reports, typical medical practice and historical data. To a limited degree, evidence for some nutritional supplementation such as thiamine and magnesium were also substantiated on 2001 Cochrane analysis. D. OUTCOME This capstone was developed with the intention of expanding clinical and aeromedical knowledge with respect to women in aviation. The attached Clinical guideline will be submitted to ASAM (American Society of Aerospace Medical Specialists) as well as to U.S. Army aeromedical authority, with potential for on line publication and access. 23 APPENDIX A: PROPOSED AEROMEDICAL CLINICAL PRACTICE GUIDELINE Overview: Dysmenorrhea occurs when prostaglandin F2α (PG F2α) causes menstrual cramping, with or without associated symptoms of nausea headaches, anxiety, fatigue, diarrhea, and bloating. In varying degrees, it affects 45-95% of reproductive aged women, causing 3 days of incapacitating symptoms and/or up to 3 lost duty days per month in 10-15% of women with untreated dysmenorrhea. Dysmenorrhea occurs most commonly in young nulliparous women. Smoking and psychological stress are additional risk factors. The physical exam in primary dysmenorrhea is normal; abnormal examination may indicate secondary dysmenorrhea from ovarian cyst, adenomyosis, leiomyomas and, less commonly, chronic salpingitis, copper IUD use, or acquired outflow tract obstruction. First line management includes the use of non-steroidal antiinflammatory drugs (NSAIDs) and combination oral contraceptives pills (OCPs), with acetaminophen acceptable when controlling mild symptoms. OCPs may be used continuously for menstrual suppression. Estrogen-progesterone delivery is also available through the vaginal ring (NuvaRing®) and the estrogen patch (OrthoEvra®) for individuals who prefer more convenient dosing. Medroxyprogesterone acetate (DepoProvera) and progesterone containing IUDs such as the levonorgestrel IUD (Mirena®) may afford pain relief through cessation of menstruation. Other medications such as danazol, (Danocrine ®) and medroxyprogesterone acetate (Depo- Provera ®) as well as gonadotropin releasing hormone (GnRH) agonists such as leuprolide (Lupron ®) and goserelin (Zoladex ®) are available and effective in treating the symptoms of endometriosis. GnRH antagonists are not currently approved for dysmenorrhea but may have therapeutic potential. NSAIDS/NSAID equivalent medications such as acetaminophen, OCPs, progesterone containing IUDs and medroxyprogesterone acetate (Depo-Provera) are more frequently waiverable. However other treatments such as danazol (Danocrine ®) or GnRH agonists/antagonists may be considered for waiver if well tolerated and, in the case of GnRH agonists/antagonists, used in conjunction with low-dose estrogen and progesterone continuous ‘add back’ therapy. Non-traditional interventions that may be beneficial include thiamine, vitamin E and fish oil supplementation, low fat vegetarian diet, acupuncture/acupressure and TENs. (transcutaneous electric nerve stimulation) Other multi-vitamin formulations and spinal manipulation have not been proven and cannot be recommended. Rose tea may be effective, but lack of FDA oversight makes this a potentially unreliable therapy. Although not studied rigorously, tobacco cessation eliminates a risk factor for dysmenorrhea as well as a self-imposed stress for flight. Surgical therapy is the preferred treatment for patients with endometriosis related infertility. More definitive surgery, such as 24 hysterectomy and bilateral salpingo-oophorectomy, may be also effective. Aeromedical Concerns: Dysmenorrhea usually begins as low grade discomfort and may progress over hours or days to severe discomfort that is distracting. It is not normally acutely incapacitating. Menorrhagia may be associated with dysmenorrhea and can cause a gradual onset anemia. Medical therapy should consist of medications that are aeromedically acceptable, such as NSAIDs and OCPs. Due to low impact on clotting mechanisms, acetaminophen for control of mild symptoms is especially acceptable in pilots operating high-risk environments such as acrobatic instruction, urban law enforcement, search and rescue operations and extreme environmental monitoring. Oral contraceptive pills afford additional benefits in terms of pregnancy prevention, prevention of incapacitating emergencies such as ovarian torsion and ectopic pregnancy, preservation of hemoglobin and potential for menstrual suppression, However, OCPs predispose towards clot formation, especially in long duration flights and in individuals with hereditary thromophilias. Due to risk for bone density loss, medroxyprogesterone acetate and GnRH agonists/antagonists must be used with caution in pilots subject to microgravity or who have other risk factors for bone loss. GnRH agonists/antagonists should be used only in conjunction with ‘add back’ therapy. Although medroxyprogesterone acetate is convenient and reliable, it is associated with weight gain and irregular menstrual bleeding. The estrogen-progesterone patch may be associated with higher rates of thrombosis than OCPs and is not ideal for pilots with risk factors such as hereditary thrombophilas or prolonged duration flights. Potassium sparing OCPs require potassium monitoring in order to prevent electrolyte imbalance. Medical Work-up: The following consults and tests are required for proper medical evaluation leading to an aeromedical disposition: A) History of symptoms B) Gynecological evaluation report c) Report of previous treatments used d) Report of any current medications or ongoing treatments e) Hemoglobin/Hematocrit . References: ASAMS:Endometriosis. Obtained on 31 Dec 2007 from http://www.asams.org/guidelines/Completed/NEW%20Endometriosis.htm. Bouchard, Philippe. 2005. GnRH antagonists: Present and Future. Ann Urol (Paris). Oct; 39 Supple S56-8. Dawood, M. Yusoff, 2006. Primary Dysmenorrhea Advances in Pathogenesis and Management. Obstet Gynecol. 108 (2), 428. 25 Ferri, Fred, 2007. Ferri’s Clinical Advisor: Instant Diagnosis and Treatment, 9th Edition. Mosby Elsevier. Philadelphia, PN. French, Linda, 2005. Dysmenorrhea. Am Fam Physician. 71 (2), 285-91. National Guideline Clearinghouse: Medical management of endometriosis. Obtained on 19 Dec 2007 from http://www.guideline.gov/summary/summary.aspx?doc_id=3961 Proctor, Michelle, Farquhar, Cynthia, 2006. Diagnosis and management of dysmenorrhoea, BMJ. 332, 1134-1138. 26 REFERENCES AERO, 2007. U.S. Army Aeromedical Resource On-line, 2007. (https://www.rucker.amedd.army.mil/flightsurgeons.html. Accessed 03 November 2007. AFP, 2007. American Family Physician. SORT. http://www.aafp.org/online/en/home/publications/journals/afp/afpsort.html. Accessed 27 December 2007. American Airlines, 2007. Corporate Information. http://www.aa.com/content/amrcorp/corporateInformation/facts/female pilots.html. Accessed 02 November 2007. ASAMS, 2007. http://www.asams.org/guidelines/Completed/NEW%20Endometriosis.htm. Accessed 29 December 2007. Bays H., 2006. Clinical overview of Omacor: a concentrated formulation of omega-3 polyunsaturated fatty acids. Am J Cardiol. 98, 71-76. Bleeker, M., Hopman, M., Rongen, G., Smits, P., 2004. Unilateral lower limb suspension can cause deep venous thrombosis. Am J Physiol Regul Integr Comp Physiol. 286, 11761177. Brown, W., Dohme, J., Wick, D.,1980. An Evaluation of Minority and Female Performance in Army Rotary Wing Aviation Training. Vol I Executive Summary. Research Report 1318. U.S. Army Research Institute for Behavioral and Social Sciences. Approved for public release. Burton, R., Whinnery, J., 2002. Biodynamics: Sustained Acceleration. In DeHart and Davis; Fundamentals of Aerospace Medicine. 3ed Edition. Lippincott Williams & Wilkins. Philadelphia, PA. Dawood,Y., 2006. Primary Dysmenorrhea: Advances in Pathogenesis and Management. Obstet Gynecol.108, 428. Dessole S., Farina M., Rubattu G., Cosmi E., Ambrosini G., Nardelli G., 2003. Sonovaginography is a new technique for assessing rectovaginal endometriosis. Fertil 27 Steril. 79, 1023-1027. Deutch B., 1995. Menstrual pain in Danish women correlated with low n-3 polyunsaturated fatty acid intake. Eur J Clin Nutr. 49, 508–516. Ebell, M., Siwek, J., Weiss, B., Woolf, S., Susman, J., Ewingman, B., Bowman, M., 2004. Strength of Recommendation Taxonomy (SORT): A Patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 69, 548-556. Eby, G., Davis, D., Halcomb, W., 1984. Reduction in duration of common colds by zinc gluconate lozenges in a double-blind study, Antimicrob Agents Chemother. 25, 20–24. Eby, G., 2007. Zinc treatment prevents dysmenorrhea. Med Hypotheses. 69, 297-301. Edelman, A., Gallo, M., Jensen, J., Nicholas, M., Schultz, K., Grimes, D., 2005. Continuous or extended cycle versus cyclic use of combined oral contraceptives for contraception. Cochrane Database of Systemic Reviews, 2005;(3) CD004695. http://www.cochrane.org/reviews/en/ab004695.html Date of last update 25 March, 2005. Accessed 04 March 2008. Edwards, R. 2004. ABC of smoking cessation. The problem of tobacco smoking. BMJ. 328, 217-219. Exacoustos C., Zupi E., Carusotti C., Rinaldo D., Marconi D., Lanzi G., Arduini, D., 2003. Staging of pelvic endometriosis: Role of sonographic appearance in determining extension of disease and modulating surgical approach. J Am Assoc Gynecol Laparosc. 10, 378-382. FAA. U.S. Department of Transportation Federal Aviation Administration. Administrator’s Handbook, August 2006. http://www.faa.gov/about/office_org/headquarters_offices/aba/admin_factbook/media/Au gust_2006_Fact_Book.pdf. Accessed 02 November 2007. Facchinetti F., Sgarbi L., Piccinini F., Volpe A., 2002. A comparison of glyceryl trinitrate with diclofenac for the treatment of primary dysmenorrhea: an open, randomized, crossover trial. Gynecol Endocrino. 16, 39-43. Feener, D., 2001. In Stenchever; Comprehensive Gynecology. 4th Edition. Mosby Elsevier. Philadelphia, PA Ferri, F., 2007. Ferri’s Clinical Advisor: Instant Diagnosis and Treatment. 9th Edition. Mosby Elsevier. Philadelphia, PN. French, L., 2005. Dysmenorrhea. Am Fam Physician. 71, 285-291. 28 Goei, G., Ralston, J., Abraham, G., 1982. Dietary patterns of patients with premenstrual tension. J Appl Nutr. 34, 1–8. Gold Standard, 2007. Elsevier. http://www.goldstandard.com/about.htm. Accessed through http://home.mdconsult.com, 02 November 2007. Grow, M., 1942. Fit to Fly, a Medical Handbook for Fliers. Appleton-Century Co. Publishers. New York, NY. Guide for Medical Examiners. 2006. Version 5. Item 41. www.faa.gov/about/office_org/headquarters_offices/avs/offices/aam/ame/guide. Item 40, p. 90. Accessed 18 December 2007. Guide for Medical Examiners, Tutorial. 2006. Physiology-Self Medication. Section 11.3.7. http://www.faa.gov/other%5Fvisit/aviation%5Findustry/designees%5Fdelegations/design ee%5Ftypes/ame/tutorial/section2/ap%5Fphysiology. Date of last update 28 November 2006. Accessed 26 December 2007. Guide for Medical Examiners Tutorial. 2006. Physiology- Alternative/Complementary Medicine. Section 11.3.9. http://www.faa.gov/other%5Fvisit/aviation%5Findustry/designees%5Fdelegations/design ee%5Ftypes/ame/tutorial/section2/ap%5Fphysiology. Date of last update 28 November 2006. Accessed 26 December 2007. Harel, Z., 1996. Supplementation with omega-3 polyunsaturated fatty acids in the management of dysmenorrhea in adolescents. Am J Obstet Gynecol. 174,4. Hazelrigg, S., 2003. Secondary spontaneous pneumothorax. Chest. 124, 781-782. Hoffman, R., Benz, E., Shattil, S., Furie, B., Silberstein, L., McGlave, P., Hematology: Basic Principles and Practice. 4th Edition. 2005. Churchill Livingstone. Philadelphia, PA. Holtz, R., 1941. Should Women Fly During the Menstrual Period? J Av Med. 12,302. Jick, S., Kaye, J., Russmann, S., Jick, H., 2006. Risk of nonfatal venous thromboembolism in women using a contraceptive transdermal patch and oral contraceptives containing norgestimate and 35 microg of ethinyl estradiol. Contraception. 73, 223-228. Lobo, R., 2007. Endometriosis: Etiology, Pathology, Diagnosis, Management. In Katz; Comprehensive Gynecology. 5th Edition. Mosby Elsevier. Philadelphia, PA. Lyons, T., 1993. Women in the Fast Jet Cockpit – Aeromedical Considerations. Aviat. Space Envion Med. 63, 814. 29 Marjoribanks, J., Proctor, M., Farquhar, C., 2003. Cochrane Database Syst Rev 2001;(2):CD001751. http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD001751/abstract.ht ml. Accessed 01 August 2007. Mahutte, N., Arici, A., 2003. Medical management of endometriosis-associated pain. Obstet Gynecol Clin North Am. 30, 133-150. Mauer E., Schall J., Mendez F., 1958. Chronic recurring spontaneous penumothorax due to endometriosis of the diaphragm. JAMA. 168, 2013-2014. Mohler, S., 2002. Acupuncture helps in treating medical problems. Flight Saf. 49, 1-4. Monserud, N., 1945. Medical Considerations: Women Airforce Service Pilots (WASP) Program. Air Surgeon's Office, HQ/AAF. 141, 28-30. Murnane, L., 2007. Legal Impediments to Service: Women in the military and the Rule of Law. Duke J Gender Law & Policy. May 2007. http://www.law.duke.edu. Accessed 18 December 2007. National Guideline Clearinghouse, 2007. www.guideline.gov. Accessed 19 December 2007. NGC/ACOG, 2004. Medical management of endometriosis.http://www.guideline.gov/summary/summary.aspx?doc_id=3961&nbr= 003099&string=endometriosis. Date of last update December 2004. Accessed 27 December 2007. NGC/Nurses Professional Association, 2003. Cyclic perimenstrual pain and discomfort: nursing management. Evidence-based clinical practice guideline. http://www.guideline.gov/summary/summary.aspx?doc_id=7199&nbr=004302&string= dysmenorrhea. Accessed 28 December 2007. Nicoletti, A., Tonelli, M., 2005. Counseling teens and young women on Depo-Provera. J Pediatr Adolesc Gynecol. 18, 297-298. Olive, D., 2007. Endometriosis. In Rakel Conn’s; Current Therapy. 59th Edition. Saunders Elsevier. Philiadelphia, PA. Ortho Evra. 2006. Manufacturer Information, www.orthoevra.com. Drug Safety, The Risk of Venous Thromboembolism, Myocardial Infarction, and Ischemic Stroke Among Women Using the Transdermal Contraceptive System Compared to Women Using Norgestimate-containing Oral Contraceptives with 35 mcg Ethinyl Estradiol, June 2006 (study unpublished with data on file at Johnson & Johnson) Accessed 03 November 30 2007. Prasad, A., Fitzgerald, J., Bao, B., Beck, F.,Chandrasekar, P., 2000. Duration of symptoms and plasma cytokine levels in patients with the common cold treated with zinc acetate. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 133, 245– 252. Press, R., Bauer K., Kujovich J., Heit J., 2002. Clinical utility of factor V Leiden (R506Q) testing for the diagnosis and management of thromboembolic disorders. Arch Pathol Lab Med.126,1304. Proctor, M., Murphy P., 2001. Herbal and dietary therapies for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2001;(2):CD002124. http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD002124/abstract.ht ml. Accessed 01 August 2007. Proctor, M., Smith, C., Farquhar, C., Stones, R., 2002. Transcutaneous electrical nerve stimulation and acupuncture for primary dysmenorrhoea. Cochrane Database of Sys Rev 2002;(1):CD002123.Date of last update 22 November 2001. Accessed 01 August 2007. Proctor M., Farquhar C., 2002. Dysmenorrhoea. Clin Evid.7,1639-1653. Proctor M., Farquhar C., Sinclair O., Johnson N., 2004. Surgical interruption of pelvic nerve pathways for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2004(3):CD001896. http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD001896/abstract.ht ml. Accessed 01 August 2007. Proctor M., Hing W., Johnson T., Murphy P., 2004. Spinal manipulation for primary and secondary dysmenorrhoea. Cochrane Database Syst Rev 2004(3):CD002119. http://www.mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD002119/abstract.ht ml. Accessed 01 August 2007. Proctor, M., Farquhar, C., 2006. Diagnosis and management of dysmenorrhoea, BMJ. 332, 1134-1138. Proctor M., Murphy P., Pattison H., Suckling J., Farquhar C., 2008. Behavioural interventions for primary and secondary dysmenorrhoea. Cochrane Database of Systematic Reviews 2007;(3) CD002248. http://mrw.interscience.wiley.com/cochrane/clsysrev/articles/CD002248/frame.html. Date of last update 01 April 2007. Accessed 04 March 2008. Rittweger, J., Winwood, K., Seynnes, O., Maarten de Boer, D., Wilks, R., Rennie, M., Naric, M., 2006. J Physio. 577, 331-337. 31 Shulman L., Nelson A., Darney P., 2004. Recent developments in hormone delivery systems. Am J Obstet Gynecol. 190, 39-48. Stratton P, Winkel C, Premkumar A, Chow C, Wilson J, Hearns-Stokes R. Heo, S., Merino, M., Nieman, L., 2003. Diagnostic accuracy of laparoscopy, magnetic resonance imaging, and histopathologic examination for the detection of endometriosis. Fertil Steril. 79,1078-1085. Swica, Y., 2007. The Transdermal Patch and the Vaginal Ring: Two Novel Methods of Combined Hormonal Contraception. Obstet Gynecol Clin North Am. 34, 31-42. Taiwan.T., 2005. Rose tea for relief of primary dysmenorrhea in adolescents: a randomized controlled trial. J Midwifery Womens Health. 50, 51-57. U.S. Army Aeromedical Policy Letters, 2006. Flight Surgeon’s Aeromedical Checklists. Last Revision 01 March 2006. Compiled by Justin Woodson. https://aamaweb.usaama.rucker.amedd.army.mil/AAMAWeb/p4.html. Accessed 03 November 2007. Ussher, M., West, R, Steptoe, A., McEwen, A., 2003. Increase in common cold symptoms and mouth ulcers following smoking cessation. Tob Control. 12, 86-88. Van Wagenen, K. 1990. Those Wonderful Women in Their Flying Machines: The Unknown Heroines of World War Two. Four Directions Press. New York, NY. Wang C., Harris W.S., Chung M., Lichtenstein A.H., Balk E.M., Kupelnick B., Jordan H.S., Lau J., 2006. N-3 fatty acids from fish or fish-oil supplements, but not -linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: A systematic review. Am J Clin Nutr. 84, 5-17. Wennberg, J., Gittelsohn, A., 1973. Small Area Variations in Health Care Delivery. Science.182, 1102 – 1108. Whitehead, R., 1935. Physical Standards for Aircraft Pilots. J Av Med. September Edition, 83. Whitehead, R., 1934. Notes from the Department of Commerce: Women Pilots. J Av Med. June Edition, 47-49. Ziaei, S., Rajaei, L., Faghihzadeh, S.Lamyian, M., 2002. Comparative Study and evaluation of side effects of low-dose contraceptive pills administered by the oral and vaginal route. Contraception 65, 329-331. 32 VITA Nicole Powell-Dunford was born on 19 February 1975 in Albany New York to Richard and Danielle Powell. She attended Albany High School and the State University of New York at Albany. earning a B.A. in English and Biology. Nicole subsequently graduated from the Uniformed Services University of the Health Sciences and completed a family medicine residency at Tripler Army Medical Center. She has served as a Combat Aviation Brigade Surgeon in the 25th Infantry Division, an Aviation Medicine Clinic Chief as well as a Task Force flight surgeon in Iraq and Afghanistan. She is married to Mike Dunford and is the proud parent of Julia and Joshua Dunford. 33