Exam #2 Review - Villanova University
advertisement

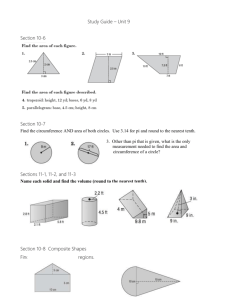
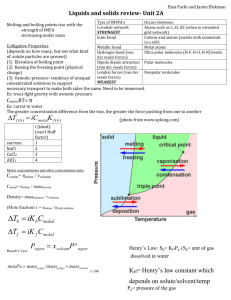
Chemistry 1152 Exam #2 Study Guide Exam will have 100 points. You will be allowed one hour to complete the exam. The exam will be a mix of multiple-choice and show your work problems, following the format of the quizzes and mid-term on the homepage. Review your quizzes, homework problems, class notes, instructor’s homepage, Chang’s web site and textbook. Do not forget to bring a functioning calculator !!! Chapter 11 1. Identify coordination numbers for atoms in simple cubic, face-centered cubic and bodycentered cubic structures. 2. Determine the number of atoms contained in a unit cell for simple cubic, face-centered cubic and body-centered cubic structures. 3. Use geometric concepts to relate a, the edge length of a unit cell, to r, the radius of atoms, in simple cubic, face-centered cubic and body-centered cubic structures. 4. Know what fraction of a unit cell is occupied by atoms, and show that the face-centered cubic structure has the closest packing for simple cubic, face-centered cubic and bodycentered cubic structures. 5. Perform calculations involving crystal structure, density, atomic radius and the number of atoms per unit cell. 6. Describe how x-ray diffraction is used to determine geometric parameters of solids. 7. Discuss the following: evaporation sublimation condensation deposition molar heat of vaporization molar heat of sublimation molar hear of fusion phase changes boiling point critical temperature and pressure melting point 8. Use Clausius – Clapeyron equation to solve for vapor pressure at a specific temperature given associated data. 9. Sketch a typical heating curve and identify various aspects of it. 10. Use phase diagrams to identify what phase(s) is/are present given specific conditions. Chapter 12 1. 2. 3. 4. Use the terms dilute unsaturated, saturated and supersaturated to describe solutions. Predict relative solubilities given the net dipole moment of solute and solvent. Define the terms miscible and immiscible. Define, determine and inter-convert between each of the following: Molarity mole fraction percent by mass molality 5. State Henry’s law and use it to determine the solubility of gases in liquids. 6. Rationalize why two common materials (NH3 or CO2) when dissolved in water do not follow Henry’s law. 7. Define colligative properties and give four examples (vapor – pressure lowering, freezing – point lowering, boiling – point elevation and osmotic pressure). 8. Use Raoult’s law to find vapor pressures or concentrations of solutions. 9. Predict the plot of pressure versus mole fraction for an ideal solution. 10. Rationalize the possibility of either positive or negative deviations from Raoult’s law by non-ideal solutions. 11. Perform calculations involving boiling-point elevation, freezing-point depression, Kf, Kb and molality. 12. Perform calculations involving osmotic pressure and molarity. 13. Use the concepts of colligative properties to determine molar mass. 14. Define the van’t Hoff factor and demonstrate how it is incorporated into the colligative property equations. 15. Give examples of common types of colloids and describe the dispersing medium and dispersed phase for each. 16. Describe the Tyndall effect. 17. Identify hydrophilic and hydrophobic colloids and describe the cleansing action of soap. Chapter 13 1. Distinguish between average and instantaneous rates of chemical reactions. 2. Define rate constant. 3. Use the concepts of stoichiometry to write reaction rate expressions in terms of the disappearance of reactants and the appearance of products. 4. Be able to identify the reaction orders, given a rate law. 5. Use rate data to determine rate laws and rate constants (know your units !!). 6. Sketch the rate of reaction versus concentration of reactant for zero and first order reactions. 7. Determine the time required for the concentration of a reactant to change a desired amount given the initial concentration and the rate constant for a first order reaction. 8. Be able to identify the order of a reaction by the units of the rate constant and be able [A]o kt to solve first-order kinetic problems given the following equation: ln [A] Happy studying !! Good Luck !! CHEM 1152 – Exam #2 – Summer 2007 – Dr. Thrush Name: ________________________________________________________ July 16, 2007 INSTRUCTIONS: Clearly print your name on the top line. Point values are given for each problem. For all multiple choice questions, please circle the correct answer. Show all work – partial credit will be given. Be sure to give all numerical answers in the proper number of significant figures and the correct units. Potentially Useful Information: 1 atm = 760 torr = 760 mm Hg = 760 torr K = °C + 273.15 No = 6.022 x 1023 . R = 0.08206 L atm/(mole.K) = 8.314 J/(mole.K) = 0.008314 kJ/(mol.K) P Hvap 1 1 ln 2 R T1 T2 P1 1 year = 31,557,600 s 1 year = 365.25 days [A]o ln kt [A] 1 day = 24 hours 1 hour = 60 min 1 min = 60 s As a community committed to the Augustinian ideals of truth, unity and love, Villanova University prides itself on maintaining the highest standards of academic integrity and does not tolerate any forms of academic dishonesty or misconduct. Accordingly, each student who takes an examination is expected to sign the following statement. I, ______________________________________ (sign your name), have not had any unsanctioned prior access to this examination and will conduct myself in an honest manner in regard to all aspects of this examination. Unless authorized by the course instructor, I will not discuss the contents of this examination, in general or specific terms, until the examination is administered to all students. Happy studying !! Good Luck !!