Thiamine & Benzoin Condensation: Biochemistry Lab
advertisement

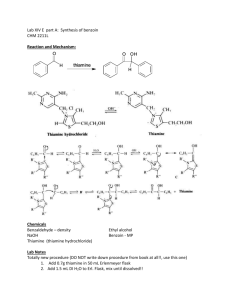
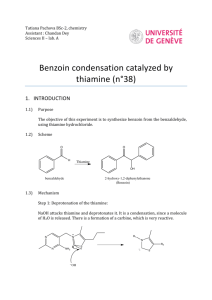
BIOCHEMISTRY LAB FUNCTION OF A COENZYME: BENZOIN CONDENSATION OF BENZALDEHYDE USING THIAMINE Coenzymes in biological systems are analogous to “reagents” in laboratory experiments. Coenzymes usually form a complex with specific enzymes, which subsequently bind the substrates. The coenzyme reacts with the substrate while they are both bound to the enzyme. Since most biochemical processes are no more than organic chemical reactions carried out under special conditions, they can be explained mechanistically, if they have been studied well enough. Enzyme-substrate-coenzyme complexes make these reactions more efficient (faster with higher yields), more selective, and more stereoselective (as well as stereospecific). Furthermore, these reactions occur at milder conditions (pH) than would normally be possible at laboratory conditions. Thiamine and its pyrophosphate derivative are coenzymes universally present in all living systems. Thiamine was originally discovered as a required nutritional factor (vitamin – like most coenzymes) in humans by its link with the disease beriberi. The deficiency of Vitamin B1 causes beriberi, which is a disease of the peripheral nervous system. Symptoms include pain and paralysis of the extremities, emaciation, or swelling of the body. The disease is most common in locations in the world, where people suffer from malnutrition. pyrophosphate pyrimidine ring N O N NH 2 O S N P O O O P O O H thiazole ring Thiamine pyrophosphate Thiamine serves as a coenzyme for three important types of enzymatic reactions: 1. Formations of acyloins (-hydroxy ketones –as in benzoin condensation) O O 2 R thiamine R R H OH 2. Nonoxidative decarboxylations of -keto acids O R 3. O CO2 thiamine R H + CO2 Oxidative decarboxylations of -keto acids O R O CO 2 thiamine O2 R H + CO 2 General Mechanism of thiamine mediated reactions: The reactive site of thiamine is the thiazole ring which undergoes deprotonation at the C-2 position under basic conditions to form a stabilized conjugate base. Due to the charge on the trisubstituted ring nitrogen (pyridine like), the conjugate acid is an ylide. The sulfur atom plays an important role in stabilizing the ylide. R' R R' S N R N S R O H ylide R' R' H B HO H S N R N S HO H B R' R N H O R' B H S O R N H R O OH H O R' S N S + OH benzoin Procedure: Add 1.0 g thiamine hydrochloride to the vial supplied. Dissolve the solid in 1.4 mL of water by sonicating the vial. Add 10 mL of 95% ethanol and sonicate the solution until it is homogeneous. To this solution add 3.0 mL of an aqueous sodium hydroxide solution (2 M). Sonicate the vial until the bright yellow color fades to a pale yellow color. Carefully measure 3.0 mL of benzaldehyde and add it (with a syringe) to the flask. Sonicate the vial until it is homogenous. Put the screw top on the flask and allow it to stand in a dark place until the crystals form. It is likely that this process will not take place earlier than two-three days. Page 2 Exercises: 1. Propose a mechanism for the non-oxidative decarboxylation of -keto acids. Given below is the first step between pyruvic acid and thiamine. Show a step by step mechanism and give the final products. R' R N S O CO 2 Page 3 2. Propose a mechanism for the oxidative decarboxylation of -keto acids. The substrate is again pyruvic acid; however another cofactor, lipoic acid, is utilized. Start with the third step shown below to give a step by step outline and the final products. R' R N HO S H B S S CO 2 lipoic acid 3. What makes the above reaction oxidative? In your answer compare the oxidation states of the products in question 1 and question 2. What is the oxidizing agent in the reaction as shown above? Page 4 4. How do you think lipoic acid could be attached to an enzyme. Think of an enzyme where lipoic acid could be covalently attached to the enzyme. Comment on a possible specific amino acid residue instrumental in the attachment. 5. Do you expect thiamine to be phosphorylated (in its pyrophosphate form) or unphosphorylated when it forms a complex with an enzyme? Explain. 6. Propose amino acid residues for the active site of the enzyme for question 1. Consider also protonating species which might be taking part in the process. Page 5