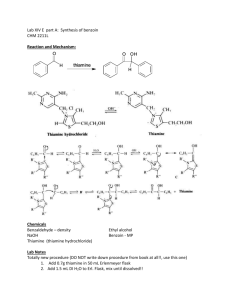
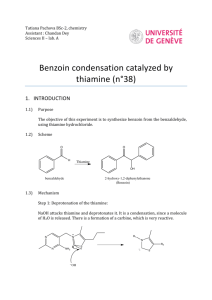
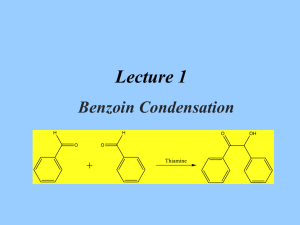
Experiment 19: Synthesis of Benzoin catalyzed by Coenzyme Spring Organic Chemistry II North Dakota State University Reaction Benzoin condensation reaction: two benzaldehyde molecules combine to form benzoin. A catalytic (12 mol %) amount of thiamine hydrochloride is used. Cl N Catalyst= H3C CH3 N N pyrimidine NH2 S OH thiazole ________________________________________________ Old method to synthesize benzoin: O OH H [CN] H2O/ EtOH O Cons: 1. Not applicable to aliphatic aldehydes. 2. NaCN is poisonous!! 2 Thiamine Hydrochloride • The hydrochloride salt form of thiamine, a vitamin essential for aerobic metabolism, cell growth, transmission of nerve impulses and acetylcholine synthesis. • Thiamine hydrochloride is similar to thiamine pyrophosphate (TPP), a coenzyme present in all living systems. 3 Ylides • A compound with positive and negative formal charges on adjacent atoms. • Thiamine hydrochloride catalyst contains an ylide • C-2 proton is very acidic – and easily removed P CH2 very acidiic 4 Reaction mechanism NH2 N S N NH2 N H R1= Thiamine N N OH O R2= -CH2CH2CH3 R3= Me R3 N R2 OH S O R1 H nucleophilic attack Ph O Ph O Benzoin HO S Ph N R1 S R2 R2 N R1 R3 R3 intramolecular H transfer intramolecular H transfer OH Ph OH O S S Ph N R1 R2 R2 N R1 R3 R3 O Ph H nucleophilic attack 5 Procedure • Dissolve thiamine hydrochloride in water • It is a salt, so it should readily dissolve with swirling • Add 95% EtOH, and cool in ice water bath • Add cold 2M NaOH dropwise through condenser (why cold 2M NaOH? Remember definition of molarity?) • Add benzaldehyde through condenser • Add boiling stone (role?), heat GENTLY at reflux 60 min (use heating mantle, no H2O bath). • There should be very little bubbling Why is it important to use ‘fresh’ benzaldehyde in this experiment? Because benzaldehye can undergo slow aerobic oxidation to benzoic acid which will cause protonation of Thiamine Deactivation of catalyst!! 6 Procedure (contd.) • Slowly cool to induce crystallization • Vacuum filter, wash with COLD water (why?) • Product should be dried on Büchner funnel • Obtain crude appearance and yield. • Recrystallize from 95% EtOH • Use absolute minimum amount of warm EtOH to dissolve, and cool slowly • Use a Büchner funnel to filter again, and dry product • Obtain yield, melting point 7