AP CHEMISTRY – Source: 1999 AP Exam, Also Data Base of MC
advertisement

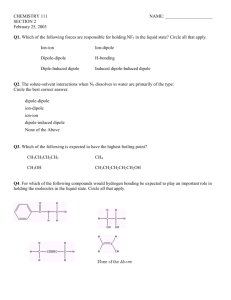
AP CHEMISTRY – Source: 1999 AP Exam, Also Data Base of MC Questions from Prior AP Exams CHAPTER 10 PRACTICE TEST CHEMISTRY SECTION I NO CALCULATORS MAY BE USED WITH SECTION I Part A Directions: Each set of lettered choice below refers to the numbered statement immediately following it. Select the one lettered choice that best fits each statement and then fill in the corresponding oval on the answer sheet. A choice may be used once, more than once, or not at all in each set. Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the corresponding oval on the answer sheet. 1. Use these answers for questions 1 - 4. (A) hydrogen bonding (D) resonance (B) hybridization (E) van der Waals forces (London dispersion forces) (C) ionic bonding 1. Is used to explain why iodine molecules are held together in the solid state E 2. Is used to explain why the boiling point of HF is greater than the boiling point of HBr A 3. Is used to explain the fact that the four bonds in methane are equivalent B 4. Is used to explain the fact that the carbon-to-carbon bonds in benzene, C6H6, are identical D 5. The structural isomers C2H5OH and CH3OCH3 would be expected to have the same values for which of the following? (Assume ideal behavior.) (A) Gaseous densities at the same temperature and pressure (B) Vapor pressures at the same temperature (C) Boiling points (D) Melting points (E) Heats of vaporization 6. CH3CH2OH boils at 78 °C and CH3OCH3 boils at - 24 °C, although both compounds have the same composition. This difference in boiling points may be attributed to a difference in CH 10 ZUMDAHL (A) molecular mass (D) hydrogen bonding (B) density (E) heat of combustion (C) specific heat 7. X = CH3-CH2-CH2-CH2-CH3 Y = CH3-CH2-CH2-CH2-OH Z = HO-CH2-CH2-CH2-OH Based on concepts of polarity and hydrogen bonding, which of the following sequences correctly lists the compounds above in the order of their increasing solubility in water? (A)Z < Y < X (B) Y < Z < X (C) Y < X < Z (D) X < Z < Y (E)X < Y < Z 8. The electron-dot structure (Lewis structure) for which of the following molecules would have two unshared pairs of electrons on the central atom? CH 8 ZUMDAHL (A) H2S (B) NH3 (C) CH4 (D) HCN (E) CO2 9. Which of the following molecules has a dipole moment of zero? CH 8 ZUMDAHL (A) C6H6 (benzene) (B) NO (C) SO2 (D) NH3 (E) H2S 10. I2(g) + 3 Cl2(g) ---> 2 ICl3(g) According to the data in the table below, what is the value (A) - 870 kJ (D) + 450 kJ (B) - 390 kJ (C) + 180 kJ (E) + 1,260 kJ Bond I---I Cl---Cl I---Cl Average Bond Energy (kj/mole) 150 240 210 11. On a mountaintop, it is observed that water boils at 90°C, not at 100°C as at sea level. This phenomenon occurs because on the mountaintop the A) equilibrium water vapor pressure is higher due to the higher atmospheric pressure B) equilibrium water vapor pressure is lower due to the higher atmospheric pressure C) equilibrium water vapor pressure equals the atmospheric pressure at a lower temperature D) water molecules have a higher average kinetic energy due to the lower atmospheric pressure E) water contains a greater concentration of dissolved Gas 12. A measured mass of an unreactive metal was dropped into a small graduated cylinder half filled with water. The following measurements were made. GENERAL INFO Mass of metal = 19.611 grams Volume of water before addition of metal = 12.4 milliliters Volume of water after addition of metal = 14.9 milliliters The density of the metal should be reported as (A) 7.8444 grams per mL (B) 7.844 grams per mL (C) 7.84 grams per mL (D) 7.8 grams per mL (E) 8 grams per mL 13. The critical temperature of a substance is the (A) temperature at which the vapor pressure of the liquid is equal to the external pressure (B) temperature at which the vapor pressure of the liquid is equal to 760 mm Hg (C) temperature at which the solid, liquid, and vapor phases are all in equilibrium (D) Temperature at which liquid and vapor phases are in equilibrium at I atmosphere (E) lowest temperature above which a substance cannot be liquified at any applied pressure 14. Which of the following statements is always true about the phase diagram of any one-component system? (A) The slope of the curve representing equilibrium between the vapor and liquid phases is positive. (B) The slope of the curve representing equilibrium between the liquid and solid phases is negative. (C) The slope of the curve representing equilibrium between the liquid and solid phases is positive. (D) The temperature at the triple point is greater than the normal freezing point. (E) The pressure at the triple point is greater than 1 atmosphere. 15. Which of the following is true at the triple point of a pure substance? (A) The vapor pressure of the solid phase always equal the vapor pressure of the liquid phase. (B) The temperature is always 0.01 K lower that the normal melting point. (C) The liquid and gas phases of the substance always have the same density and are therefore indistinguishable. (D) The solid phase always melts if the pressure increases at constant temperature. (E) The liquid phase always vaporizes if the pressure increases at constant temperature. 16. Use the following diagram for 16-18. 16. The normal boiling point of the substance represented by the phase diagram above is (A) -15 °C (B) -10 °C (C) 140 °C (D) greater than 140 °C (E) not determinable from the diagram 17. The phase diagram above provides sufficient information for determining the (A) entropy change on vaporization (B) conditions necessary for sublimation (C) deviations from ideal gas behavior of the gas phase (D) latent heat of vaporization (E) latent heat of fusion Most Dense Least Dense 18. For the substance represented in the (A) Solid Gas diagram, which of the phases is most dense and (B) Solid Liquid which is least dense at - 15 °C. (C) Liquid Solid (D) Liquid Gas 19. Which of the following actions would be (E) The diagram gives no information about densities. likely to change the boiling point of a sample of a pure liquid in an open container? I. Placing it in a smaller container II. Increasing the number of moles of the liquid in the container III. Moving the container and liquid to a higher altitude (A) I only (D) II and III only (B) II only (E) I, II, and III (C) III only 20. Questions 20-23 refer to the following descriptions of bonding in different types of solids. (A) Lattice of positive and negative ions held together by electrostatic forces. (B) Closely packed lattice with delocalized electrons throughout (C) Strong single covalent bonds with weak intermolecular forces. (D) Strong multiple covalent bonds (including ∏ bonds.) with weak intermolecular forces (E) Macromolecules held together with strong polar bonds. 20. Polysaccharides, empirical formula: CH2O(s). E 21. Calcium sulfide, CaS (s) A 22. Silver, Ag B 23. Oxygen, O2(s) D 24. The cooling curve for a pure substance as it changes from a liquid to a solid is shown right. The solid and the liquid coexist at (A) point Q only (D) all points on the curve (B) point R only between R and T (C) all points on the curve (E) no point on the curve between Q and S FREE RESPONSE Calculators and Equation Tables may be used. 1a. Compare the structural models used to describe solids, liquids, and gases. Include (1) distance between particles, (2) relative strength of intermolecular forces, and (3) the extent of entropy. 1b. (i) Is vaporization an endothermic or exothermic process? Support your answer with an explanation. (ii) In what ways is the high heat of vaporization of water important to each of us personally? 2006 Question6:Answer each Qin terms of principles of molecular behavior & chemical concepts. (a) The structures for glucose, C6H12O6, and cyclohexane, C6H12, are shown below. Identify the type(s) of intermolecular attractive forces in: (i) pure glucose Answer: Hydrogen bonding OR dipole-dipole interactions OR van de Waals interactions. (London dispersion forces may also be mentioned.) (ii) pure cyclohexane Answer: London dispersion forces (b) Glucose is soluble in water but cyclohexane is not soluble in water. Explain. Answer: Glucose is polar and cyclohexane is nonpolar. • Polar solutes (such as glucose) are generally soluble in polar solvents such as water. • Nonpolar solutes (such as cyclohexane) are not soluble in the polar solvent. Students generally answered Part a of this question correctly. Students seemed to have trouble differentiating between intermolecular and intramolecular forces throughout this question. 2005 Question 7: Use principles of atomic structure, bonding and/or intermolecular forces to respond to each of the following. Your response must include specific information about all substances referred to in each question. (a) At a pressure of 1 atm, the boiling point of NH3(l) is 240 K, whereas the BP of NF3(l) is 144 K. (i) Identify the intermolecular force(s) in each substance. Answer: NH3 has dispersion forces and hydrogen-bonding forces. NF3 has dispersion forces and dipole-dipole forces. (Credit earned for hydrogen bonding and dipole-dipole forces.) (ii) Account for the differences in the boiling points of the substances. Answer: The higher boiling point for NH3 is due to the greater strength of the hydrogen-bonding intermolecular attractive forces among NH3 molecules compared to that of the dipole-dipole attractive forces among NF3 molecules.