SOAPS AND DETERGENTS
advertisement

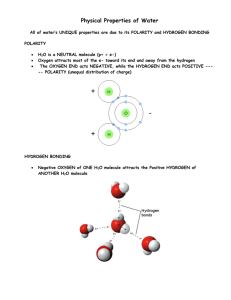
NAME:___________________________ DATE:_____________ 2.5 What’s so special about water? Instructor AHS Ms. Kasia Room 109 UNIT 2 CHEMICAL BONDING: COVALENT BONDING 2.5 What’s so special about water? AIM: WATER (H2O) Water is a remarkable substance. This lab will help you understand some of the water’s properties and the following passage describes water’s special properties in terms of molecular structure. You will learn about its surface tension properties and its ability to dissolve compounds (such as sugar and salt). Vocabulary: Surface Tension: is a property of the surface of a liquid that allows it to resist an external force Hydrogen Bonding: force of attraction between water molecules Intermolecular Forces: a type of force between covalent molecules (hydrogen bonding is an example) LAB: WHEN IS A GLASS OF WATER REALLY FULL? HYPOTHESIS: If ________________is affected by ___________________ then_________________________. It's often hard to tell. A glass full of water is like a bus at rush hour-you can usually squeeze a bit more into it. Surprise yourself by finding out how much more you can put into a full glass of water. MATERIALS Beaker Coins Water Bin PROCEDURE 1. Fill a glass right to the top with water. 2. Gently start dropping coins into the water. (It's best to hold the coins on edge and slip them into the water.) 3. You'll notice that the top of the water bulges out above the top of the glass. How many coins can you add before the water overflows? Record your observations in the Data Collection and Analysis. DATA COLLECTION AND ANALYSIS Resources:http://www.ontariosciencecentre.ca/scizone/homelab/fullglass.asp, So what happened? Water molecules have a strong attraction for one another. This attraction is due to hydrogen bonding, which is an example of intermolecular forces. Hydrogen bonding occurs between molecules in which hydrogen is bonded to fluorine OR oxygen OR nitrogen. In the case of water, these intermolecular forces act between molecules of H2O. Inside the glass, the molecules that are surrounded by other molecules of water are attracted in all directions. But the molecules at the surface have no water above them, so they are strongly attracted downwards by the molecules below them. These attractive forces are strong enough to keep the water from spilling over the top of the glass, even when the level rises quite a bit beyond it. But eventually the volume of water above the rim of the glass becomes too great for the surface tension to hold, and the water will spill. REFLECTION Directions: In a paragraph summarize the findings of your experiment. Make sure to explain the following 1. Why did the water not spill easily when adding pennies. 2. What is surface tension 3. What are the attractive forces between the water molecules. STOP AND DRAW Directions: Draw a representation of what happened in the glass. Your drawing should represent the way you understand the reading on this page. (HINT: Draw H2O molecules, show how they interact, show what is happening at the surface of the cup) Resources:http://www.ontariosciencecentre.ca/scizone/homelab/fullglass.asp, When you are done with the experiment and Reflection…. Directions: Complete the reading on the next page. Answer the questions that are aligned with the reading. The special properties of water The special properties of water can be related to its molecular structure, which governs the way its molecules interact with each other and with other substances. QUESTIONS 1. Why is it important to study the molecular structure of water? In water, the bond between Hydrogen and Oxygen is a polar covalent bond. The molecular structure that water takes on is asymmetrical (uneven). Because of both of these factors, water is a polar molecule. The oxygen in water really wants electrons, so it pulls them away from each of the hydrogens. This makes the oxygen atom negatively charged and the hydrogen atoms positively charged. 2. What does “polar molecule” mean? The polarity of the water molecule and its ability to form hydrogen bonds allow water to dissolve, soften or swell organic substances. That is why it can easily dissolve, for example, soap or sugar or salt. Dissolving Ionic Compounds Ionic compounds such as sodium chloride (NaCl) will dissolve in polar solvents but no in non-polar solvents. The positive ion of NaCl is attracted to the partially negatively charged atom in the polar solvent molecule. The negative ion of the solute (NaCl) is attracted to the partially positively charged atom on the solvent molecule. Intermolecular forces and their effects The strong attraction between the water molecules, hydrogen bonding, makes it have a high boiling point of 100oC. That is because it takes a lot of energy to cause the molecule of water to disperse (separate) into the atmosphere as a gas. The tendency of water molecules to cling to one another means that a lot of energy is needed to move them apart. That also means that water does not flow as easily as other liquids. Resources:http://www.ontariosciencecentre.ca/scizone/homelab/fullglass.asp, 3. Which is the partially negatively charged atom in H2O, hydrogen or oxygen? 4. Which is the partially positively charged atom in H2O, hydrogen or oxygen? Resources:http://www.ontariosciencecentre.ca/scizone/homelab/fullglass.asp,