FUNCTIONAL GROUPS
advertisement
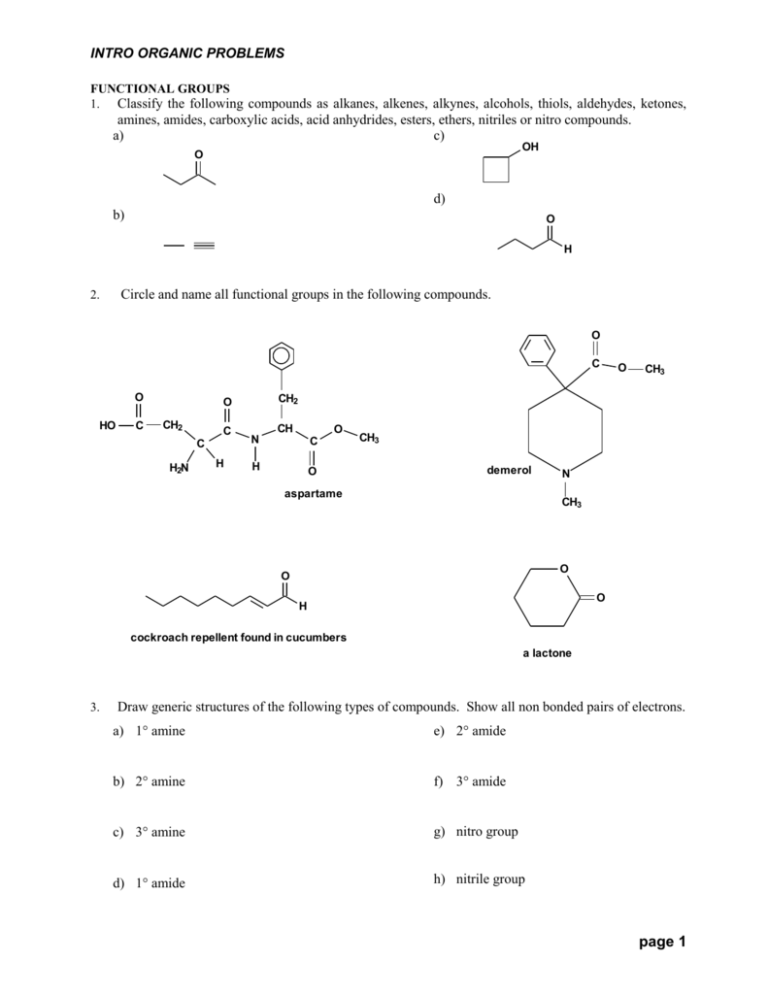
INTRO ORGANIC PROBLEMS FUNCTIONAL GROUPS 1. Classify the following compounds as alkanes, alkenes, alkynes, alcohols, thiols, aldehydes, ketones, amines, amides, carboxylic acids, acid anhydrides, esters, ethers, nitriles or nitro compounds. a) c) OH O d) b) O H Circle and name all functional groups in the following compounds. 2. O C O HO C C C H2N H CH3 CH2 O CH2 O CH O N C H O CH3 demerol aspartame N CH3 O O O H cockroach repellent found in cucumbers a lactone 3. Draw generic structures of the following types of compounds. Show all non bonded pairs of electrons. a) 1 amine e) 2 amide b) 2 amine f) 3 amide c) 3 amine g) nitro group d) 1 amide h) nitrile group page 1 INTRO ORGANIC PROBLEMS 4. 5. 6. Draw generic structures of the following types of compounds. Show all non bonded pairs of electrons. a) ether d) alcohol b) sulfide e) phenol c) thiol f) alkyl halide Draw generic structures of the following types of compounds. Show all non bonded pairs of electrons. a) peroxide c) aldehyde b) hydroperoxide d) ketone List 4 examples of synthetic organic compounds: ………………………………………………………………………………………………………….. 7. List 4 examples of natural organic compounds: ………………………………………………………………………………………………………….. 8. Complete the following: Approximately …………. % of fossil fuels consumed is used to make chemical products and approximately ……………….% of fossil fuels consumed is burned as energy. BONDING 9. How many bonds does carbon always have in its compounds? ……….. . Why can carbon never exceed 4 bonds? …………………………………………………………………………………… . When can carbon have 3 bonds? ……………………………………………………………………… . 10. The number of bonds a Group A atom forms to become isoelectronic with the nearest noble gas is called its …………………….. . 11. Define ‘isoelectronic’. …………………………………………………………………….. 12. Draw Lewis symbols of phosphorus, oxygen, bromine, silicon and boron atoms. 13. What is the relationship between the Group number and the number of valence electrons for the representative elements? ……………………………………………… 14. Define ‘valence’ electrons. …………………………………………………………………………… . 15. Draw the Lewis structure of the hydrides of sulfur, boron, and nitrogen. page 2 INTRO ORGANIC PROBLEMS FUNCTIONAL GROUPS 16. Identify the type of compound shown in the following structures. Indicate degree of substitution, i.e., 1, 2, or 3 where applicable. CH3 CH3 N N H O O N C C N N CH3 H O N C H 17. Circle the functional group(s) in the following structures and state the type. CH2 18. OH O COOH CH3COOC(CH3)3 Circle the functional groups and state the types. O CH3 C O H2N COOH H CH3CHCH2CH2OCCH3 in banana oil in fruit pits fruit ripening hormone causes leaves to drop in the autumn limonene (in limes) 19. Write a suitable structure for: a) two alcohols with the formula C3H8O b) an ether with the formula C3H8O c) and aldehyde with the formula C3H6O d) a cyclic ketone with the formula C4H6O ELECTRONEGATIVITY 20. Explain in your own words, the meaning of ‘electronegativity’. ………………………………… ………………………………………………………………………………………………………….. page 3 INTRO ORGANIC PROBLEMS 21. Without looking at Linus Pauling’s table of electronegativity, rank the atoms in the following clusters in order of increasing EN, where 1 = lowest, 3 = highest. Describe the periodic trends in EN in each case. a) F Cl Br b) N O F c) Li Na K d) Be B C 22. State the value of EN for pure covalent bonds …………. . Give the formula of 3 pure covalent, non elemental, binary comounds (made from two different atoms). …………………………………….. 23. Write the upper and lower limits of EN and give one example each of a binary compound for the following: a) non polar covalent compound b) polar covalent compound c) ionic compound 24. Write the symbols meaning ‘partial + charge’ and ‘partial – charge’. 25. The separation of + and – charge creates a ………………….. . 26. Explain the term ‘inductive effect’ as it applies to an OH bond. 27. Use Linus Pauling’s table of EN to calculate the EN in the bonds of the following compounds and classify each, i.e., polar, non polar, etc. a) NaBr b) BeBr2 c) NCl3 28. Write out the ground state electron configuration of the valence shell of each noble gas and explain the importance of these configurations with respect to the chemical behavior of the representative elements. 29. Give the order of decreasing ionization energy for the following, using 1 = lowest, 4 = highest. a) transition metals, non metals, alkali metals, metalloids 30. Give the name of the theory that means that pairs of electrons move as far apart as possible in a bonded molecule. ……………………………………………………………………………….. BONDING 31. Write out the complete ground state electron configuration of carbon. page 4 INTRO ORGANIC PROBLEMS 32. A single bond is also called a ……………….. bond. a) A double bond is made of 1 ……………….. bond and 1 …………………. bond. b) A triple bond is made of 1 ………………… bond and 2 …………………. bonds. HYBRIDIZATION 33. State the hybridization state of carbon in the following situations: a) C has four bonds ……………. b) C has three bonds and one bond …………… c) C has two bonds and two bonds. …………… 34. Write the hybridization state of each atom under the conditions shown in the following table: C N O H Halogen only bonds (no bonds) one bond (1 double bond) ---------- two bonds (2 double or 1 triple) ---------- 35. State the hybridization state of the circled atoms in the following compounds: H Br O H C CH2 O C CH3 C N H H F H CH H3C 36. CH3 O H3C C H3C N HC H2C C N H What is true about the hybridization state of H in all of its compounds? …………………………. 37. When are halogens unhybridized? …………………………………………………………………. 38. Give the hybridization state of C in: a) a methyl cation ……………….. b) a methyl radical ……………….. c) a methyl anion ……………….. page 5 INTRO ORGANIC PROBLEMS 39. A compound containing an alternating pattern of single and double (or triple) bonds is said to have ……………………………. unsaturation. When double (or triple) bonds are separated by more than one single bond the compound is said to have ……………………………. unsaturation. When double or triple bonds are adjacent, the compound is said to have ………………………… unsaturation. CARBOCATION STABILITY 40. Carbocation stability increases as follows; methyl, phenyl, vinyl, 1, 2 and 3, with allyl and benzyl having about the same stability as a 2 carbocation. Draw structures of these carbocations in order of increasing stability. (Allyl is slightly less stable and benzyl is slightly more stable than 2 carbocations). 41. Explain the carbocation stability order of 3 > 2 > 1 > methyl. (Hint: there are 2 reasons). ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… 42. List two means by which carbocations are stabilized by adjoining alkyl groups. ……………………………………………………………………………………………………… LEWIS STRUCTURES 43. Draw Lewis structures of phosgene (COCl2), hydrazine (N2H4) and formaldehyde (CH2O). page 6 INTRO ORGANIC PROBLEMS 44. Draw Lewis structures for iodous acid (HIO2), sodium dihydrogen arsentate (NaH2AsO4), and HS-. 45. Draw the Lewis structure of nitromethane (CH3NO2) and calculate the formal charge on the C, the N and both O atoms in the structure. 46. Name and draw the Lewis symbols of the following bonded ions: 47. a) a cation of bromine …………………………………. b) a cation of oxygen …………………………………. c) an anion of carbon ………………………………….. List 3 atoms, which after bonding, usually have less than 8 valence electrons. Also give an example of a bonded structure for each. 48. Define ‘hypervalent’ and draw Lewis structures of three compounds in which the central atom is hypervalent. page 7 INTRO ORGANIC PROBLEMS RESONANCE 49. Draw all possible different resonance structures of a) a benzyl cation, b) a benzyl anion, c) an allyl cation. Show electron movement between structures by pushing electrons with curved arrows. 50. Explain why resonance increases the stability of ions. ………………………………………. …………………………………………………………………………………………………………. ACIDITY/BASICITY 51. An acid producing a proton (H+ ion) in water is called ………………………………….. acid. Define Arrhenius base and give an example of one. …………………………………………………………. . …………………………………………………………………………………………………………. 52. Define ‘Bronsted acid’. Define Bronsted base and give an example of one that is not an Arrhenius base. ……………………………………………………………………………………………………. …………………………………………………………………………………………………………. 53. Acid/base pairs that differ by a proton are called ………………………… acid/base pairs. 54. In the following table, write in the missing conjugate acids and bases and calculate their pK values. Recall pKa + pKb = 14 for all conjugate acid/base pairs. Refer to tables of pK values only as a check. Bronsted acid pKa CH3COOH 4.7 15.74 HAsO4-2 pKb OHH2BO3- 4.8 CO3-2 3.7 11.5 3.1 HSO4- Bronsted base NO2- 2.0 page 8 INTRO ORGANIC PROBLEMS 55. Write the formula and give the pKa of the strongest acid that can exist in water …………………… , and the strongest base that can exist in water. ………………………………….. . 56. Explain what is meant by the expression ‘water is a leveling solvent’? ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… ……………………………………………………………………………………………………… 57. Calculate pKeq and refer to your pKeq graph to determine extent of the forward reaction (% completion) for the following acid/base reactions. Fill in missing formulas as well. % pKeq Acid Base Conj. Base Conj. Acid HBr NaHCO3 NaBr H2CO3 HN3 KF KN3 H2SO4 H2O 58. For what values of pKeq and Keq is a reaction said to ‘go to completion’? ……………….., …………. 59. Answer true or false. (Wrong answers subtracted from correct answers. Do not guess.) a) An acid will donate a H+ to a base whose conjugate acid is stronger. ………….. b) A base will accept a H+ from an acid whose conjugate base is weaker ………….. 60. Define ‘Lewis acid’ and give an example of two that are not Bronsted acids. ………………… …………………………………………………………………………………………………………… 61. Define ‘Lewis base’ and give two examples. ……………………………….………………….. …………………………………………………………………………………………………………… NUCLEOPHILES/ELECTROPHILES 62. The definition of an electrophile is the same as that of a Lewis acid and their periodic trends for binary acids are exactly the same. The definition of a nucleophile is the same as that of a Lewis base but their periodic trends are not exactly the same. Describe the difference. (See page 38 in the notes). …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… …………………………………………………………………………………………………………… 63. Write the formula of a good nucleophile that is also a strong Bronsted base (low pKb) ……………. . Write the formula of a good nucleophile that is a weak Bronsted base (high pKb) ………………. . Write the formula of a good electrophile that is also a strong Bronsted acid (low pKa) ………………. page 9 INTRO ORGANIC PROBLEMS 64. Electrophilic atoms/ions can be recognized in molecules because they are +, or have empty orbitals to receive electron pairs, or are + (and have a good leaving group that can leave to make room for a new bond from a nucleophile). Nucleophilic atoms/ions can be recognized in molecules because they are -, -, or have loosely held electrons to donate, e.g., non bonded electrons, electrons in weak bonds (especially C to C bonds). In the following structures, identify all nucleophiles and electrophiles. Where possible indicate if the species is good, fair or poor. H R H H C C R R H X H H H arene H C R C O O H R aldehyde H alcohol alkene O O R alkyl halide C H 2º amine R R N H H H alkane R H R C O C R R S H thiol acid anhydride ACIDS/BASES 65. Without looking in pK tables, in each group below, number the compounds in order of increasing acidity, where 1 = least acidic and 4 = most acidic. a) HBr HF HCl HI b) OH2 NH3 HF CH4 c) CHF2COOH CH2FCOOH CH3COOH CF3COOH page 10 INTRO ORGANIC PROBLEMS 66. Without looking in tables, approximately how do the acidities of these pairs of acids compare i.e., by what factor do their pKa values differ ? a) H2SO3 vs. HSO3-, ………………………………. b) H3AsO4 vs. HAsO4-2 …………………………….. 67. Repeat question 66 for the following series of oxyacids: HIO, HIO2, HIO3, HIO4 ………………………………………………………………………………………………………. 68. In each group, circle the atom that is most polarizable. a) H2O H2S H2Se b) NH3 PH3 AsH3 c) F- Cl- Br- I- -Cl -F 69. In each group, circle the best leaving group. a) -I -Br b) -OH2+ -OH c) -NH3+ -NH2 70. In each group, circle the one(s) that: a) cannot be a nucleophile SO4-2 HSO4- H2SO4 Fe+3 b) cannot be an electrophile H+ OH- CH3OH H2O 71. The following mechanism is incorrect. Write the correct mechanism and state what the error was. H Cl + + - Na OH H2O + NaCl page 11 INTRO ORGANIC PROBLEMS 72. page 12




