Week 1
advertisement

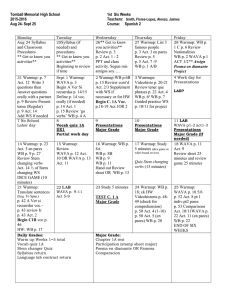
Chemistry Agenda Spring Semester: Week 1 Conservation of Matter and Stoichiometry 3. The conservation of atoms in chemical reactions leads to the principle of conservation of matter and the ability to calculate the mass of products and reactants. As a basis for understanding this concept: a. Students know how to describe chemical reactions by writing balanced equations. b. Students know the quantity one mole is set by defining one mole of carbon 12 atoms to have a mass of exactly 12 grams. c. Students know one mole equals 6.021023 particles (atoms or molecules). d. Students know how to determine the molar mass of a molecule from its chemical formula and a table of atomic masses and how to convert the mass of a molecular substance to moles, number of particles, or volume of gas at standard temperature and pressure. January 25-26 108min WarmUp: Agenda/ Modeling Chemical Reactions p320 30min Lecture: 11.1 Describing Chemical Reactions 20min InClass: Conceptual Problems 11.1-11.3 p324-328 20min InClass: Review of Final (for test on Friday) 18min InClass: Lab Procedure: Precipitation Reactions: Formation of Solids p345 20min Homework: No homework tonight! January 27 38min WarmUp: Balancing Chemical Equations 23min Lecture: 11.2 Types of Chemical Reactions 15min Homework: Read and take notes 11.1 Answer q1-5 p324-328 DUE AT THE BEGINNING OF NEXT CLASS January 28-29 108 min WarmUp: More Chemical Equations 8min Test: Most-missed final questions 20min Lab: Precipitation Reactions: Formation of Solids p345 80min Lab report due at the end of the period Homework: Read and take notes 11.2 answer q17-19p334-335 DUE AT THE BEGINNING OF NEXT CLASS Chemistry WarmUp Copy ALL of these assignments into your binder Including dates, WarmUps, InClass assignments AND page numbers! January 25-26 WarmUp: Agenda/ Modeling Chemical Reactions p320 InClass: Conceptual Problems 11.1-11.3 p324-328 InClass: Review of Final (for test on Friday) InClass: Lab Procedure: Precipitation Reactions: Formation of Solids p345 Homework: No homework tonight! January 27 WarmUp: Balancing Chemical Equations Homework: Read and take notes 11.1 Answer q1-5 p324-328 DUE NEXT CLASS January 28-29 WarmUp: More Chemical Equations Test: Most-missed final questions Lab: Precipitation Reactions: Formation of Solids p345 Lab report due at the end of the period Homework: Read and take notes 11.2 answer q17-19p334-335 DUE NEXT CLASS