Balancing Chemical Reactions Homework
advertisement
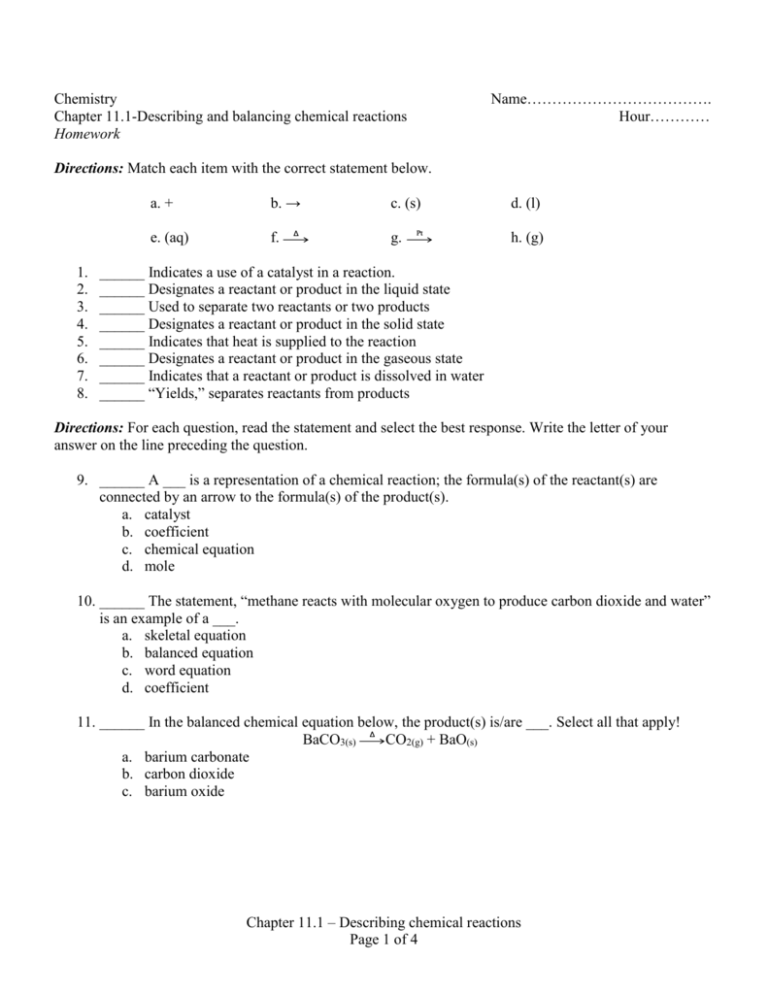
Name………………………………. Hour………… Chemistry Chapter 11.1-Describing and balancing chemical reactions Homework Directions: Match each item with the correct statement below. 1. 2. 3. 4. 5. 6. 7. 8. a. + b. → e. (aq) f. Δ c. (s) g. Pt d. (l) h. (g) ______ Indicates a use of a catalyst in a reaction. ______ Designates a reactant or product in the liquid state ______ Used to separate two reactants or two products ______ Designates a reactant or product in the solid state ______ Indicates that heat is supplied to the reaction ______ Designates a reactant or product in the gaseous state ______ Indicates that a reactant or product is dissolved in water ______ “Yields,” separates reactants from products Directions: For each question, read the statement and select the best response. Write the letter of your answer on the line preceding the question. 9. ______ A ___ is a representation of a chemical reaction; the formula(s) of the reactant(s) are connected by an arrow to the formula(s) of the product(s). a. catalyst b. coefficient c. chemical equation d. mole 10. ______ The statement, “methane reacts with molecular oxygen to produce carbon dioxide and water” is an example of a ___. a. skeletal equation b. balanced equation c. word equation d. coefficient 11. ______ In the balanced chemical equation below, the product(s) is/are ___. Select all that apply! BaCO3(s) Δ CO2(g) + BaO(s) a. barium carbonate b. carbon dioxide c. barium oxide Chapter 11.1 – Describing chemical reactions Page 1 of 4 12. ______ In the balanced chemical equation below, the reactant(s) is/are ___. Select all that apply! 2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l) a. carbon dioxide b. water c. dicarbon hexahydride d. oxygen 13. ______ A chemical equation that indicates the relative amounts of reactants and products is called a ___. a. coefficient b. skeleton equation c. balanced equation d. catalyst 14. ______ Balanced chemical equations are based on the Law of Conservation of Mass. That is, the number of atoms on the reactant side is __ the number of atoms on the product side. a. greater than b. less than c. equal to 15. ______ A ___ reaction is a chemical change in which two or more substances react to form a single new substance. a. double replacement b. single replacement c. combination d. decomposition e. combustion 16. ______ A ___ reaction is a chemical change in which a single compound breaks down into two or more simpler products. a. double replacement b. single replacement c. combination d. decomposition e. combustion 17. ______ A ___ reaction is a chemical change in which a single element or halogen replaces a second element in a compound. a. double replacement b. single replacement c. combination d. decomposition e. combustion Chapter 11.1 – Describing chemical reactions Page 2 of 4 18. ______ A ___ reaction is a chemical change in which the positive ions between two compound are exchanged. a. double replacement b. single replacement c. combination d. decomposition e. combustion 19. ______ A ___ reaction is a chemical change in which an element or compound reacts with oxygen, producing energy and often carbon dioxide. a. double replacement b. single replacement c. combination d. decomposition e. combustion Directions: Match each item with the correct statement below. a. AB → A + B d. AB + CD → AD + CB b. A + B → AB e. A + O2 → AOn + energy c. A + BC → B + AC 20. ______ General format for a combustion reaction 21. ______ General format for a double replacement reaction 22. ______ General format for a combination reaction 23. ______ General format for a single replacement reaction 24. ______ General format for a decomposition reaction Directions: Balance the following reactions and identify the reaction type. Place your balancing coefficient in the lines provided to you. 25. ______ Mg(s) + ______O2(g) → ______ MgO(s) Reaction type:_____________________________________________ 26. ______ HgO(s) → ______ Hg(l) + ______ O2(g) Reaction type:_____________________________________________ 27. ______ Ca(OH)2(aq) + ______ HCl(aq) → ______ CaCl2(aq) + ______ H2O(l) Reaction type:_____________________________________________ 28. ______ K(s) + ______ H2O(l) → ______ KOH(aq) + ______ H2(g) Reaction type:_____________________________________________ Chapter 11.1 – Describing chemical reactions Page 3 of 4 29. ______ Mg(s) + ______ N2(g) → ______ Mg3N2(s) Reaction type:_____________________________________________ 30. ______ CH4(g) + ______ O2(g) → ______ CO2(g) + ______ H2O(g) Reaction type:_____________________________________________ 31. ______ Na2S(aq) + ______ Cd(NO3)2(aq) → ______ CdS(s) + ______ NaNO3(aq) Reaction type:_____________________________________________ 32. ______ Al(s) + ______Cl2(g) → ______AlCl3(s) Reaction type:_____________________________________________ 33. ______ Fe(s) + ______ Pb(NO3)2(aq) → ______ Pb(s) + ______ Fe(NO3)2(aq) Reaction type:_____________________________________________ 34. ______Cl2(aq) + ______ NaI(aq) → ______ NaCl(aq) + ______ I2(aq) Reaction type:_____________________________________________ 35. ______ S(s) + ______ O2(g) → ______ SO3(g) Reaction type:_____________________________________________ 36. ______ Al(NO3)3(aq) + ______ (NH4)2Cr2O7(aq) → ______ Al2(Cr2O7)3(s) + ______ NH4NO3(aq) Reaction type:_____________________________________________ 37. ______ Zn(s) + ______ Cu(NO3)2(aq) → ______ Cu(s) + ______ Zn(NO3)2(aq) Reaction type:_____________________________________________ 38. ______ C6H12O6(s) + ______ O2(g) → ______ CO2(g) + ______ H2O(g) Reaction type:_____________________________________________ 39. ______ Al(OH)3(s) → ______ Al2O3(s) + ______ H2O(l) Reaction type:_____________________________________________ 40. ______ C2H6O(l) + ______ O2(g) → ______ CO2(g) + ______ H2O(g) Reaction type:_____________________________________________ 41. ______ Ba(NO3)2(aq) + ______ Na2CO3(aq) → ______ BaCO3(s) + ______ NaNO3(aq) Reaction type:_____________________________________________ Chapter 11.1 – Describing chemical reactions Page 4 of 4









