science
advertisement

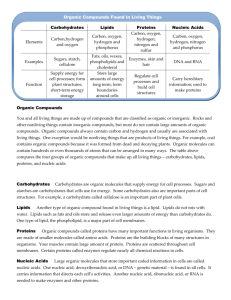
SCIENCE CHEMICAL NATURE OF CELL CLASS WORK Ch. 5 NAME ___________________ DATE ___________________ PERIOD _________________ THE CHEMICAL NATURE OF THE CELL A famous scientist was once asked to describe the nature of a cell. Everyone around him expected a long, technical answer, but all he said was that a cell is "a bag of chemicals." While this description is far from being complete, it is, nevertheless, correct as far as it goes. Cells are bags of chemicals. Within the cell the chemicals are constantly reacting with one another. The sum total of these reactions, called the cell's metabolism, consists of a tremendous number of very specific reactions, each being a part of a metabolic pathway. Through the reaction in these pathways, the needs of the cell (and organism) are met. There are two important differences between a living cell and a "bag of chemicals." The first is that the contents of the cell are organized. The second is that the chemicals reactions are controlled. In the nonliving bag, the various substances would react more or less at random. What ever could happen in the bag, would happen. In the cell, substances react at closely regulated rates, and in a definite order, so as to serve the needs of the cell as a whole. In this chapter we shall study the basic chemical substances found in cells. We shall also examine the ways in which these substances are essential to the cells and to the organism as a whole. ELEMENTS AND COMPOUNDS: An element is a pure substance that cannot be broken down into simpler substances by ordinary chemical means. There are more than one hundred known elements, but the materials of the cell are made up almost entirely of only four of these - hydrogen, carbon, oxygen, and nitrogen. Table 5-1 lists the important elements that are found in cells: 1 Compounds are formed when two or more elements combine chemically. For convenience, compounds are divided into two basic types: inorganic and organic. Inorganic compounds are usually found in the nonliving world, but some of them are needed by living organisms. Various inorganic acids, bases, and salts are found in cells, but by far the most important inorganic compound in cells is water. Life cannot continue for any great length of time without water. Depending on the cell type, from 70 to 80 percent of cellular material is water. This means that if you weigh 100 pounds, 70 or more of those pounds is water. Water is a very unusual substance. Its unique properties enable water to serve as the medium in which the chemical processes of life actually take place. Water plays a major role in the chemical breakdown and buildup of cellular compounds. It is an excellent solvent, and materials cannot pass through living membranes unless they are dissolved in water. Finally, water plays an important part in temperature regulation in warm-blooded animals. Organic compounds are any compounds that include the elements carbon and hydrogen. However, other elements may be present as well. Most of the compounds of the cell (and organism) are organic. Table 5-2 lists the most important types of cellular organic compounds. Most of the molecules of the organic compounds in cells are large, but they are usually made up of varying numbers of smaller units, which we can think of as building blocks. Such compounds can generally be broken down into their original building blocks. The building blocks of all organic compounds are not the same. Each type of organic compound has its own specific kind of units, as we shall see in the following discussion. CARBOHYDRATES: Carbohydrates are organic compounds that are made up of carbon, hydrogen, and oxygen. If we burn a carbohydrate, such as table sugar, a black residue remains. This residue is carbon. In burning, the hydrogen and oxygen molecules form water and are released as water vapor. This leaves only the black carbon residue. One of the most common carbohydrates is the simple sugar glucose. Its chemical formula is C6H12O6 , which means that a molecule of glucose contains 6 atoms of carbon, 12 atoms of hydrogen, and 6 atoms of oxygen (see Figure 5-1). 2 Glucose is one of the major building blocks of carbohydrates. A cell can make many kinds of carbohydrates by putting together various numbers of glucose units in various ways. The synthesis of carbohydrates generally involves a type of reaction called dehydration synthesis. For example, when two molecules of glucose are brought together in the presence of certain enzymes, one molecule loses a hydrogen atom (H), and the other loses a hydroxyl group (OH). The H and OH combine to form water, and the two glucose molecules are joined together at the sites where the H and OH were lost. This type of reaction is called dehydration synthesis because it involves the elimination of water (see Figure 5-2). When carbohydrates are broken down in the course of cell metabolism, glucose molecules (or other building blocks) are the end products. The breakdown of carbohydrates occurs by hydrolysis, which is the reverse of dehydration synthesis. In hydrolysis, the elements of water, H and OH, are returned to the glucose molecules. Complex sugars and starches as well as cellulose, the major ingredient of plant cell walls, are all carbohydrates. The names of most carbohydrates end in ose. Carbohydrates provide the cell with its main source of energy. When glucose is oxidized during respiration, energy is released. 3 LIPIDS: Lipids, or fats, are another class of organic compounds made up of carbon, hydrogen, and oxygen. However, liquids contain relatively less oxygen than carbohydrates, and their chemical structure is quite different. Figure 5-3 shows a fat molecule made from glycerol and fatty acids. Lipids contain more energy than carbohydrates and are often stored by cells as a reserve energy supply. Lipids are also a basic structural part of all cell membranes. Thus, they play a part in regulating the passage of materials into and out of the cell and its organelles. PROTEINS: Proteins are the most numerous group of organic compounds in a cell. They are huge, complicated molecules that generally contain carbon, hydrogen, oxygen, and nitrogen. Phosphorus and sulfur may also be present. (An easy way to remember the elements of proteins is to think of the name P S COHN.) Amino acids (see Figure 5-5) are the building blocks of proteins. Most protein molecules consist of several hundred amino acid units joined together. Like carbohydrates, proteins are formed by dehydration synthesis, and they are broken down by hydrolysis. 4 There are about 20 different kinds of amino acids found in the cell. Primarily, it is the sequence of amino acids - that is, the order in which they are joined together - that determines the kind of protein that is made. Proteins are important in the growth and repair of cells, and they also make up structural elements in the body. These substances can also be used as a source of energy. Enzymes are a special group of proteins, which are found in all cells. They control the rate at which chemical reactions take place. Without enzymes, the rapid reactions necessary for the life processes, such as respiration, would be impossible. NUCLEIC ACIDS: Nucleic acids are the largest of the organic molecules found in cells. One of the nucleic acids, DNA (deoxyribonucleic acid), is found primarily in the nucleus. The other nucleic acid, RNA (ribonucleic acid), is found both in the nucleus and in the cytoplasm. There are several types of RNA, and each serves a distinct function. Nucleotides are the building blocks of nucleic acids. Some DNA molecules may contain more than 20,000 nucleotides. The information that an organism inherits from its parent is coded in the sequence of the thousands of nucleotide units (see Figure 5-4). 5 From the preceding discussion we can see that the cell does indeed have a chemical nature. Reaction take place in cells 24 hours a day. Unlike most factories or other businesses, cells do not shut down in the evening or take vacations. Living organisms require constant attention. It might be well for us to remember that each of the types of compounds we have mentioned is itself nonliving. However, when put together and organized into the substance of a cell, the quality of life emerges. The ability of organisms to live successfully in the world comes, in large measure, from the ability of their cellular materials to react correctly and continuously. 6