Chapter 3 Notes Section 3.1
advertisement

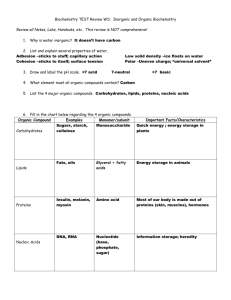
Chapter 3 Notes Section 3.1 -­ Chemistry of Life Matter -­ Anything that has mass and takes up space Energy -­ Anything that brings about change Atoms 3 Particles of an Atom Proton -­ Positive Charge -­ in the nucleus Neutron -­ No Charge -­ in the nucleus Electron -­ Negative Charge -­ outside the nucleus Elements -­ Made of only one kind of atom -­ Cannot be broken down Elements in the Human Body O = Oxygen C = Carbon H = Hydrogen N = Nitrogen Ca = Calcium P = Phosphorus K = Potassium S = Sulfur Na = Sodium Cl = Chlorine Mg = Magnesium Compounds -­ Made of 2 or more elements -­‐ Cannot be physically taken apart -­‐ Example – salt – NaCl 2 Types of Compounds 1) Molecular – made of molecules – Example – water – H2O 2) Ionic – made of positive and negative ions – Example – salt – NaCl Ions – atoms with a positive or a negative charge Mixture – combination of substances in which individual substances retain their own properties – Ex – fruit salad Organic Compounds – always contain Carbon and Hydrogen and are associated with living things Ex -­ Carbohydrates, Lipids, Proteins, and Nucleic Acids Carbohydrates -­‐ organic -­‐ supply energy -­‐ Examples – sugars and starches Lipids -­‐ do not mix with water -­‐ release more energy than carbs -­‐ Phospholipid is a major part of the cell membrane -­‐ Examples – fats and oils Nucleic Acids -­‐ large organic molecules -­‐ store coded info -­‐ Ex – DNA – genetic material found in all cells RNA -­ needed to make enzymes and proteins Inorganic Compounds -­‐ DO NOT contain carbon -­‐ Example – Water – H2O Importance of Water 1) Living things need water to survive 2) Need water to grow and reproduce 3) Needed for chemical reactions and to transport materials