Compound and mixture notes 2 (self) Answer
advertisement
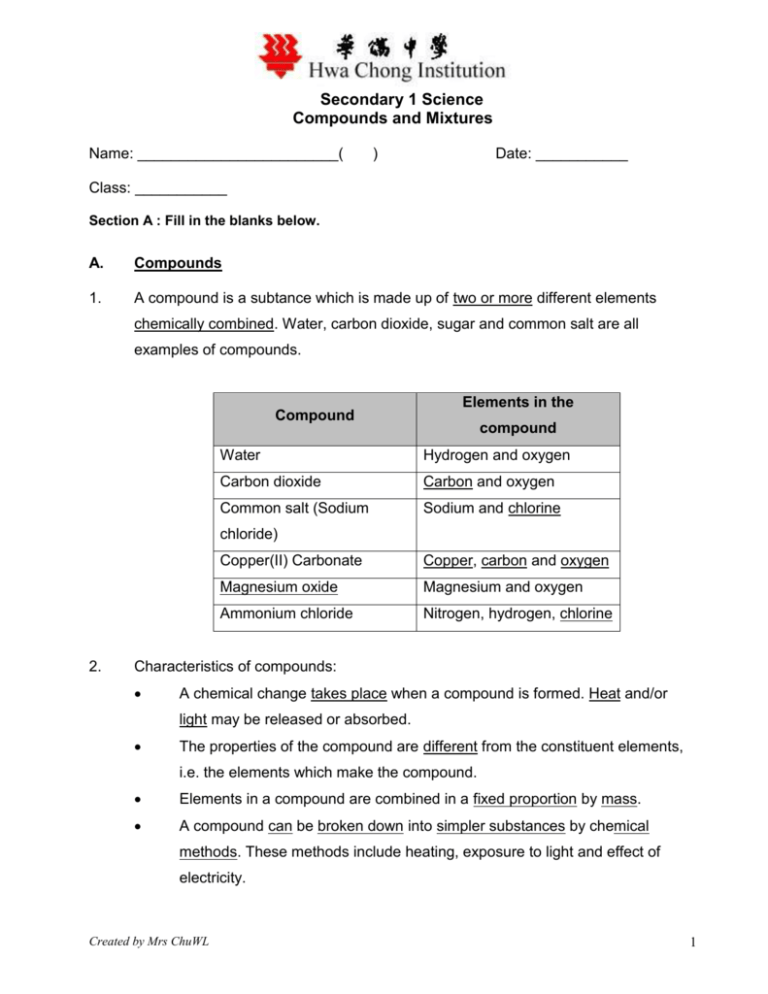
Secondary 1 Science Compounds and Mixtures Name: ________________________( ) Date: ___________ Class: ___________ Section A : Fill in the blanks below. A. Compounds 1. A compound is a subtance which is made up of two or more different elements chemically combined. Water, carbon dioxide, sugar and common salt are all examples of compounds. Compound Elements in the compound Water Hydrogen and oxygen Carbon dioxide Carbon and oxygen Common salt (Sodium Sodium and chlorine chloride) 2. Copper(II) Carbonate Copper, carbon and oxygen Magnesium oxide Magnesium and oxygen Ammonium chloride Nitrogen, hydrogen, chlorine Characteristics of compounds: A chemical change takes place when a compound is formed. Heat and/or light may be released or absorbed. The properties of the compound are different from the constituent elements, i.e. the elements which make the compound. Elements in a compound are combined in a fixed proportion by mass. A compound can be broken down into simpler substances by chemical methods. These methods include heating, exposure to light and effect of electricity. Created by Mrs ChuWL 1 B. Mixtures 1. A mixture consists of two or more substances, which are not chemically combined. Sugar solution, salt solution, soil, air, aerated water, blood are examples of mixtures. 2. Characteristics of mixtures: When a mixture is formed, no chemical reaction occurs. Hence, there is little or no light or heat taken in or given out. A mixture has the properties of the substances of which it is made. A mixture can be separated easily by physical methods, for example, filtration, distillation, evaporation. C. The components in a mixture are not mixed in any fixed proportion. Word Equation ( This section will be discussed further in class) 1. Word equations represent chemical reactions which take place. 2. Word equation can tell us what substances are reactants or the starting materials, and what substances are product(s). 3. Products are new substances obtained from the reaction. Section B : Answer the following questions in the space provided. 1. Explain why water is a compound. Water is made up of oxygen and hydrogen. Oxygen is a colourless gas which supports combustion while hydrogen is a gas which burns with a pale blue flame. Water is a liquid at room temperature and does not have the properties of its constituents elements. Also, water contains hydrogen and oxygen, combined in a fixed proportion. For example, 1 g of hydrogen gas + 8 g of oxygen 9 g of water. Lastly, water can only be broken down by chemical means, i.e. electrolysis (passing through electricity). Created by Mrs ChuWL 2 2. Explain why air is a mixture. Air can be made by simply mixing its component gases, nitrogen, oxygen, carbon dioxide, noble gases and water vapour. Air has properties of its constituents, nitrogen, oxygen, carbon dioxide, water vapour and noble gases. Air support combustion because the oxygen gas in it supports combustion. Air can be separated into its components by fractional distillation. We can obtain nitrogen, and oxygen separately by physical means. The constituents gases in air may be mixed in different proportions, This may vary with place and time. For example, we can find a higher proportion of carbon dioxide in the city than at the seaside (why?) 3. Distinguish between elements, compounds and mixtures. Element Compound Mixture An element is made up of Different elements in a The components in only one kind of atom. compound combine in a mixtures are not mixed in fixed proportion by mass. any fixed proportion. An element is the simplest A chemical change takes No chemical reaction pure substances place when a compound occurs. There is little or is formed. no heat and/or light taken in or given out. An element has properties A compound has A mixture has the similar to those of their properties which are properties of the atoms. different from the substances of which it is properties of its made. constituent elements. Elements cannot be A compound can only be A mixture can be broken down whether by broken down, by chemical separated easily by physical or chemical methods, for example physical methods, for means. heating (decomposition) example, filtration, or by passing electricity distillation, evaporation. through it. Created by Mrs ChuWL 3 4. Complete the following word equation: a. Oxygen + Hydrogen water b. Carbon + Oxygen (in excess) Carbon dioxide c. Magnesium + oxygen Magnesium oxide Created by Mrs ChuWL 4








