Moles of Iron and Copper Post Lab Handout
advertisement

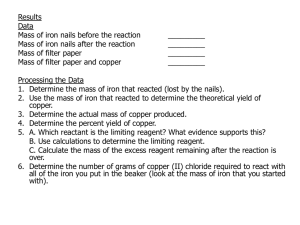
Moles of Iron and Copper Post Lab – Lab Write-up due ____________________ Analysis 1. . Find the following masses by subtraction of the data measurements: a. mass of Iron that reacted b. mass of copper that was produced – the actual yield 2. Write a balanced chemical equation for the reaction that occurred. Consider any Iron ions to be Iron II 3. Determine the limiting reactant and the theoretical yield of Copper in grams note: Copper II Chloride is a hydrate that contains 2 water molecules. The weight of the two waters must be added to the formula mass. 4. Determine the percent yield of Copper Discussion 1. If your percent yield was not 100% explain why. 2. How would you know if some of the Copper II Chloride was left in the beaker? 3. Some percent yields will be over 100% How is this possible? 4. Compare your experimental results to the balanced equation. Do they correlate ? Explain any deviation. Moles of Iron and Copper Post Lab – Lab Write-up due ____________________ Analysis 1. . Find the following masses by subtraction of the data measurements: a. mass of Iron that reacted b. mass of copper that was produced – the actual yield 2. Write a balanced chemical equation for the reaction that occurred. Consider any Iron ions to be Iron II 3. Determine the limiting reactant and the theoretical yield of Copper in grams note: Copper II Chloride is a hydrate that contains 2 water molecules. The weight of the two waters must be added to the formula mass. 4. Determine the percent yield of Copper Discussion 1. If your percent yield was not 100% explain why. 2. How would you know if some of the Copper II Chloride was left in the beaker? 3. Some percent yields will be over 100% How is this possible? 4. Compare your experimental results to the balanced equation. Do they correlate ? Explain any deviation.