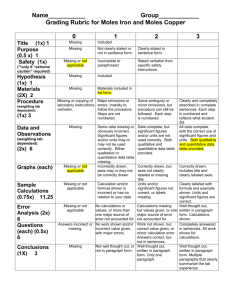
Moles of Iron and Copper Lab Data Sheet (type all answers) Data
advertisement

Moles of Iron and Copper Lab Data Sheet (type all answers) Data Before the Reaction: Color of copper II chloride solution________________________________________________ Mass of empty, dry beaker _______________________(g) Mass of beaker + copper II chloride _______________________(g) Mass of two iron nails _______________________(g) After the Reaction: Color of the solution remaining in beaker____________________________________________ Mass of filter paper _______________________(g) Mass of two iron nails _______________________(g) Mass of filter paper + copper (dry) _______________________(g) Calculations (SHOW ALL WORK FOR FULL CREDIT) 1. Mass of iron used in the reaction 2. Mass of copper II chloride used 3. Mass of copper metal produced 4. Moles of iron used 5. Moles of copper produced 6. Atoms of iron used 7. Atoms of copper produced 8. Calculate the ratio of moles of copper produced to moles of iron used. Simplify to smallest numbers. 9. Was there any evidence that some of the copper II chloride solution was left in the beaker? Explain your answer. 10. What is the name of the type of reaction you just observed? 11. Write the balanced equation for the reaction. (yes, you must predict the products formed, one of them is an iron II compound) 12. Compare the mole ratio from your calculations (Q #4 & 5) to the mole ratio from the balanced equation. Explain your results briefly.