CHEM 1311A EXAM I A FALL 2001
advertisement
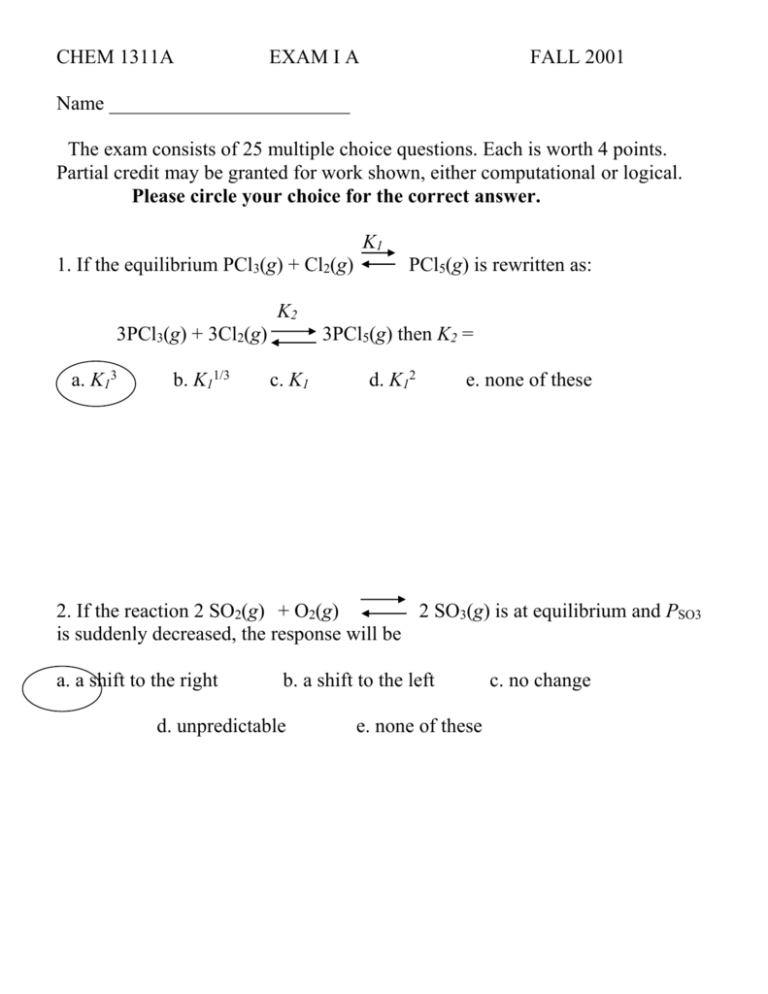
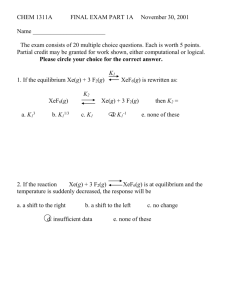
CHEM 1311A EXAM I A FALL 2001 Name ________________________ The exam consists of 25 multiple choice questions. Each is worth 4 points. Partial credit may be granted for work shown, either computational or logical. Please circle your choice for the correct answer. K1 1. If the equilibrium PCl3(g) + Cl2(g) PCl5(g) is rewritten as: K2 3PCl3(g) + 3Cl2(g) a. K13 b. K11/3 3PCl5(g) then K2 = c. K1 d. K12 e. none of these 2. If the reaction 2 SO2(g) + O2(g) 2 SO3(g) is at equilibrium and PSO3 is suddenly decreased, the response will be a. a shift to the right b. a shift to the left d. unpredictable e. none of these c. no change 3. The reaction 2 NO(g) + Br2(g) 2 NOBr(g) has K = 116.6 @ 25oC. Using the following partial pressures, calculate the reaction quotient and chose the direction the reaction will proceed. PNO = 0.105 atm PBr2 = 0.400 atm PNOBr = 0.969 a. 21.3, left b. 213, left c. 213, right d. 169, right e. none of these 4. Two buffer solutions are prepared using H2S and HS- (pKa = 7.04). The first has [H2S] = 0.15 M and [HS-] = 0.12 M with a pH of 6.94. The second has [H2S] = 0.30 M and [HS-] = 0.24 M. Its pH is a. 7.04 b. 7.14 c. 8.96 d. 6.94 e. none of these 5. The pH of a solution prepared by mixing 50 mL of 0.10 M HBr with 40 mL of 0.10 M KOH is a. 3.00 b. 2.75 c. 2.35 d. 1.95 e. none of these 6. A solution of 0.500 mol of formic acid (Ka = 1.8 x 10-4) and 0.450 mol sodium formate in 500 mL of water is prepared. The equilibrium H3O+ concentration is a. 1.92 x 10-4 b. 2.00 x 10-6 c. 2.00 x 10-4 d. 2.40 x 10-5 e. none of these 7. 50 mL of 0.75 M hydrazoic acid (HN3; Ka = 1.9 x 10-5) is titrated with M NaOH solution. The pH before any base is added is a. 5.72 b. 6.86 c. 2.98 d. 2.42 e. none of these 8. The following reaction is exothermic: 3 NO(g) N2O(g) + NO2(g) Which statement is true? a. b. c. d. e. as temperature is decreased, reaction shifts right as pressure is decreased, reaction shifts right as temperature is decreased, reaction shifts left as volume is increased, reaction shifts right none of the above 0.10 9. Which of the following is a Brønsted-Lowrey acid-base pair? a. H2CO3 ; CO32- b. H3PO4 ; HPO42- c. HCl ; NaOH d. NH4+ ; NH3 e. none of these 10. Ammonium carbamate reacts to form ammonia and carbon dioxide with the following equilibrium: NH4OCONH2(s) 2 NH3(g) + CO2(g) If a sample of the solid is sealed in an evacuated vessel and allowed to equilibrate at 25oC, the total pressure is 0.115 atm. The equilibrium constant at 25oC will be a. 2.25 x 10-4 b. 1.52 x 10-3 c. 6.08 x 10-3 d. not enough data e. none of these 11. If the pH of a 1.5 M solution of benzoic acid is 2.35, the Ka = a. 1.3 x 10-5 b. 1.3 x 10-2 c. 2.3 x 10-2 d. 2.3 x 10-5 e. none of these 12. The equilibrium expression for the following reaction: C(s) + H2O(l) + Cl2(g) COCl2(g) + H2(g) Is a. PCOCl2PH2/ [C][H2O]PCl2 c. PCl2 / PCOCl2PH2 b. [C][H2O]PCl2 / PCOCl2PH2 d. PCOCl2PH2/PCl2 e. none of these 13. A solution of 0.10 M KF will a. b. c. d. e. have a pH less than 7 due to the formation of HF. have a pH of 7 since KF does not react with water. have a pH greater than 7 due to the formation of HF. have a pH less than 7 due to an increase in [H3O+]. None of these 14. 0.01 moles of strong acid is added to a buffer of 0.08 moles of weak acid (Ka = 1 x 10-4) and 0.08 moles of conjugate base. The resulting solution will have a. b. c. d. e. 0.09 moles of weak acid and 0.09 moles of conjugate base. 0.11 moles of weak acid and 0.09 moles of conjugate base. 0.11 moles of weak acid and 0.07 moles of conjugate base. 0.01 moles of strong acid and 0.09 moles of weak acid. None of these 15. A saturated solution of benzoic acid in water has a concentration of 0.016 M. At the same temperature a saturated solution of benzoic acid in diethyl ether has a concentration of 5.41 M. The partition coefficient for the reaction benzoic acid (aq) benzoic acid (ether) is a. 2.96 x 10-3 b. 5.91 x 10-3 c. 440 d. 320 e. none of these 16. For the reaction CO(g) + Cl2(g) COCl2(g) K = 0.20 at 600oC. At equilibrium at 600oC the partial pressures of CO and Cl2 are 0.025 and 3.5 x 10-3 respectively. The partial pressure of COCl2 is a. 1.8 x 10-5 b. 1.44 x 10-4 c. 3.6 x 10-3 d. 2.5 x 10-2 e. none of these 17. For carbonic acid, H2CO3, Ka1 = 4.3 x 10-7. Therefore, Ka2 is a. 2.2 x 10-7 b. less than 4.3 x 10-7 c. greater than 4.3 x 10-7 d. 4.3 x 10-7 e. none of these 18. On the curve in question 19 (below) the point where pH pKa of the starting material is labeled a. E b. C c. B d. A e. D 19. This curve represents a titration of a a. b. c. d. e. weak acid with a strong base. weak base with a strong acid. strong acid with a strong base. strong base with a strong acid. strong base with a salt. 20. When considering the titration in problem 19, the most appropriate indicator would be ___ with its color changing over the pH range ____. a. Thymol blue; 8.0 – 9.6 b. alizarin yellow; 10.1 12.0 c. methyl red; 4.8 – 6.0 d. cresol red; 7.0 – 8.8 e. phenolphthalein; 8.2 – 10.0 21. HCl, HBr, and HI are all strong acids while HF is a weak acid. This can be explained by: a. b. c. d. e. HCl, HBr, and HI all effectively dissociate 100% in water. The HF bond is more ionic than the others. The high electronegativity of F inhibits the dissociation of HF in water. Both a and c. None of the above 22. As equilibrium in a chemical reaction is approached, a. the rate of the forward reaction approaches zero. b. the rate of the reverse reaction approaches zero. c. the rates of both the forward and the reverse reactions approach the same value d. Both a and b are correct. e. None of these 23. The value of the equilibrium constant for a particular chemical reaction is dependent upon a. b. c. d. e. the temperature of the reaction vessel. the initial amounts of reactants. the final pressure of the reaction vessel. a, b, and c none of these 24. Which of the following ions can be considered amphoteric? a. HSO4- b. H+ c. PO43- d. F- e. none of these 25. Which of the following Ka values belong to the strongest acid? a. 6.6 x 10-4 b. 4.6 x 10-4 c. 9.1 x 10-8 d. 3.0 x 10-8 e. there is insufficient data