ANTISEIZURE DRUGS
advertisement

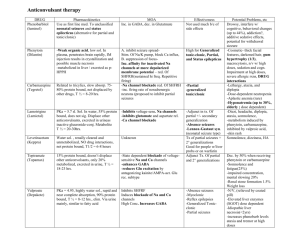
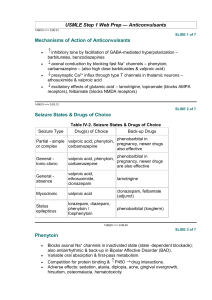
ANTISEIZURE DRUGS Dental Pharmacology Randal A. Skidgel, Ph.D. Dept. of Pharmacology HISTORY Ancient Times: “Seizure” may have originated from belief that epileptics were “seized by the devil.” “Epilepsy” is derived from the Greek “epilambanein” meaning “to seize or attack.” 1850 : Bromides discovered 1870: John Hughlings Jackson proposed first rational mechanism for seizures. 1912: First clinical use of phenobarbital to treat epilepsy 1929 - 1938: Hans Berger developed the EEG and showed the relationship between discharges in the brain and the clinical manifestations of epileptic seizures. 1938: Merritt and Putnam systematically studied 746 compounds and discovered phenytoin as effective antiseizure drug. DEFINITIONS: Seizures: result from abnormal neuronal discharge in the central nervous system produced by either focal or generalized disturbances of brain tissue. They result in abnormal phenomena of motor (convulsion), sensory, autonomic, or psychic origin. Epilepsy: A condition characterized by recurrent seizures of a particular pattern unprovoked by any immediate identified cause. Incidence = 1/300 to 1/100 Epileptic Syndromes: A cluster of symptoms frequently occurring together, including seizure types, precipitating factors, age of onset, family history, prognosis, etc.. ETIOLOGY AND MECHANISMS: Epileptic seizures are result from hyperexcitable neurons caused by: 1. 2. 3. 4. Increased activity of voltage-gated ion channels (e.g., Na+ & Ca++ channels) Decreased inhibitory (i.e., GABA) neurotransmission Increased excitatory neurotransmission (i.e., glutamate receptors) Alteration of extracellular ion concentration (e.g., potassium, calcium). 1 Classification of Seizures Frequency Loss of Consciousness PARTIAL (FOCAL) Simple partial 10 % No Complex partial 35 % Partial with secondarily generalized tonicclonic seizure Seizure Type Duration Features 20 - 60 sec Focal motor (specific muscle groups), sensory (e.g., tingling, hot or cold sensations) or speech disturbances Impaired 30 sec to 2 min Complex symptoms; confused behavior, dreamy state, amnesia; often associated with automatisms (purposeless movements). 10 % Yes 1 - 2 min Simple or complex partial seizure evolves into loss of consciousness, rigid extension of trunk and limbs (tonic), then rhythmic contraction of arms and legs (clonic). Tonic-Clonic (grand mal) 30 % Yes 1 - 5 min Loss of consciousness; massive contraction of skeletal muscle; rigid extension of trunk and limbs (tonic; posture called opisthotonos), then rhythmic contraction of arms and legs (clonic). Absence (petit mal) 10 % Impaired < 30 sec Abrupt brief onset of impaired consciousness, cessation of activities and staring. Characteristic 3 per second spike and wave pattern on EEG. Commonly in children (3 - 15 years old). Myoclonic <3% No 1 - 5 sec Brief, shocklike contraction of muscles; may be restricted to part of extremity or may be generalized. Can occur in clusters for several min. Atonic/Akinetic <2% Yes 5 sec - mins Sudden loss of postural tone leading to sagging of the head or falling. Sudden freezing of motion (called “akinetic”) 7% Yes >5 min A state of continuous seizure activity of > 5 min or 2 or more discrete seizures between which baseline consciousness is not regained. This is a medical emergency that can be fatal up to 35% of the time. GENERALIZED Status Epilepticus 2 Figure 1. Pathways of seizure spread. A, Focal seizure with spread to adjacent and contralateral cortical regions. B, Focal seizure with secondary generalization. Seizure discharge activates subcortical centers (A), which then activate entire cortex (B). C, Primary generalized absence seizure in which thalamocortical relays are believed to act on a diffusely hyperexcitable cortex. Figure 2. Relationship between cortical EEG, extracellular and intracellular recordings in a seizure focus. A, Seizure was induced by local application of a convulsant agent. The extracellular recording was made through a high-pass filter. Note the high-frequency firing of the neuron evident in both extracellular and intracellular recording during the paroxysmal depolarization shift (PDS). This seizure is representative of a partial with secondarily generalized tonic-clonic seizure. B, Recording during an absence seizure, demonstrating the characteristic generalized spike and wave discharges at a frequency of 3 per second. 3 DRUG THERAPY OF EPILEPSY AND SEIZURES Mechanisms of action of antiseizure drugs: Major known mechanisms by which antiseizure drugs work: 1. 2. 3. 4. Prolongs Inactivation state of voltage-dependent Na+ channels in a use-dependent fashion. Increase the effectiveness of inhibitory GABA transmission via the GABAA receptor. Inhibition of Ca++ currents through T-type Ca++ channels. Inhibition of excitatory glutamate transmission via ionotropic receptors. Figure 3. Prolongation of Na+ channel inactivation state by antiseizure drugs. A, resting state in which Na+ channel activation gate (A) is closed. B, Arrival of an action potential causes depolarization and opening of activation gate (A) and Na+ flows into the cell. C, As depolarization continues, an inactivation gate (B) moves into the channel. Some antiseizure drugs (black box; e.g., phenytoin) prolong the inactivated state of the channel, presumably by preventing reopening of the inactivation gate. 4 Figure 4. Enhancement of GABA transmission by antiseizure drugs. In the presence of the transmitter GABA, the GABAA receptor opens, allowing an influx of Cl-, which in turn, increases membrane polarization (hyperpolarizes), reducing the likelihood of firing of the neuron. Some antiseizure drugs (top) work by reducing the metabolism of GABA. Others act at the GABAA receptor, enhancing Cl- influx in response to GABA. Figure 5. Reduction of current through T-type Ca++ channels by antiseizure drugs. Some drugs reduce the flow of Ca++ through T-type Ca++ channels, thus reducing pacemaker current that underlies the thalamic rhythm in spikes and waves seen in generalized absence seizures. 5 Figure 6. Effect of Antiseizure Drugs on Glutamate Receptors. Antiseizure drugs that reduce sodium channel transmission will indirectly affect glutamate transmission by decreasing the amount of transmitter released from the presynaptic terminal. Felbamate and Topiramate are weak antagonists directly on the postsynaptic glutamate receptors. C. General Principles of Drug Therapy Objective: To control seizures completely without causing intolerable side effects.. Important Concepts: - Choice of drug depends on seizure type - Single drug therapy tried first - Titration of dose necessary - Monitor plasma drug concentrations - Therapy tailored to individual - Drugs treat symptoms; do not cure underlying cause -Therapeutic failure can be due to many causes (most common- poor compliance) 6 V. “CLASSICAL” DRUGS USED TO TREAT EPILEPSY AND SEIZURES (Introduced before 1979). Common Characteristics: -Absorption good -cleared by hepatic metabolism -clearance relatively slow (T1/2 > 12 hours) -complex pharmacokinetics -Side effects common -Drug interactions common VI. NEWER ANTISEIZURE DRUGS (Introduced since 1993) Common Characteristics (for those that are not derivatives of classical drugs): -Broader spectrum of activity -Fewer drug interactions -Less complex Pharmacokinetics -More expensive 7 VII. SIDE EFFECTS AND DRUG INTERACTIONS A. B. Adverse Reactions 1. Minor Reactions (common) -gastrointestinal disturbances (most drugs) -sedative effects (most drugs) -ataxia (many drugs) -visual disturbances (several drugs) -weight gain/loss (several drugs) 2. Minor Reactions (specific to drug) -gingival hyperplasia (phenytoin) -hirsutism (phenytoin) -inhibition of platelet aggregation (valproic acid) -altered behavior in children and elderly (barbiturates) 3. Serious reactions -skin eruptions (hypersensitivity) (most drugs) -blood dyscrasias (most drugs) very rare -hepatitis (phenytoin, valproic acid, carbamazepine) -alopecia (valproic acid) rare -serum sickness (immune reaction) -absence status epilepticus (rare; clonazepam + valproic acid; tiagabine?) -teratogenic (most established drugs; probably new drugs as well?) Drug Interactions 1. Decreases in Drug Plasma Concentration Produced by Enzyme Induction Some antiseizure drugs induce hepatic drug-metabolizing enzymes: Phenytoin Phenobarbital and Primidone Carbamazepine and Oxcarbazepine (less effect) This results in a decrease the plasma concentration of a variety of other drugs such as antibiotics, other antiseizure drugs, oral anticoagulants, oral contraceptives, etc. 2. Increases in Drug Plasma Concentration Produced by Enzyme Inhibition Many drugs can increase the plasma concentration of other drugs by inhibiting their metabolism (i.e., they compete for the same metabolizing enzyme). Examples: Phenytoin, valproic acid, phenobarbital and primidone are all metabolized by the same Cyt P450. 8 3. Increases in Drug Plasma Concentration by Displacement from Plasma Protein Binding Some drugs are highly bound to plasma proteins and can increase the plasma concentration of other drugs by displacing them from plasma protein binding sites. Example: Phenytoin and valproic acid are highly bound to plasma proteins and can increase the concentration of each other or drugs like diazepam that are also bound to plasma protein VIII. “Classical” Antiseizure Drugs in Clinical Use A. Phenytoin (Dilantin; hydantoin derivative; introduced in 1938) Mechanism: Slows rate of recovery of voltage-gated Na+ channels from inactivation Uses: One of the most widely used antiseizure drugs. Can be used in all types of epilepsy except absence, myoclonic or atonic seizures. Can be used i.v. to treat status epilepticus, but at <50 mg/min to prevent cardiac toxicity and CNS depression. Metabolism: 95% metabolized before excretion. Major metabolite is p-hydroxyphenyl derivative (inactive). t1/2 = 6 - 24 h. At higher concentration, half life increases to 20 - 60 h, due to saturation of metabolizing enzymes. Toxicity: Nystagmus, diplopia, vertigo, ataxia, gingival hyperplasia, hirsutism. Interactions: Induces hepatic drug metabolizing enzymes. High degree of plasma protein binding (90%) and may displace other drugs Figure 8. Relationship between dose and steady state plasma concentration of phenytoin. Results for two patients are illustrated. In both patients, there is a linear relationship between dose and plasma concentration at low doses. As the dose increases, there is a transition to a non-linear relationship. This transition occurs at different doses in each patient, illustrating the need to start at low doses and increase them gradually, tailoring the therapy to each patient. 9 B. C. Carbamazepine (Tegretol; iminostilbene derivative; approved in 1974 for use in U.S.) Mechanism: Slows reactivation of Na+ channels Uses: Partial seizures and Generalized tonic-clonic seizures. (May exacerbate absence and myoclonic seizures). Metabolism: Converted to active 10,11-epoxide and then to inactive glucuronide. t1/2 = 10 - 20 h during chronic therapy - much longer in patients after single dose. Toxicity: More toxic than most antiseizure drugs. Common side-effects include drowsiness, dizziness, ataxia, and nausea. Interactions: Induces hepatic drug metabolizing enzymes Clonazepam (Klonopin; benzodiazepine; introduced for use in epilepsy in 1960’s ) Mechanism: Enhances GABA binding to receptors, increases chloride channel opening. Indirectly (via GABA) blocks T-type Ca++ channels. Uses: Absence, myoclonic, and atonic seizures. Tolerance usually develops after 1 to 6 months of therapy. Metabolism: t1/2 = 1 day; initial i.v dose rapidly redistributes from brain into other tissues (in 1- 2 h). 99% metabolized, mainly to inactive 7-amino derivatives. Toxicity: Drowsiness, ataxia, personality changes (aggression, hyperactivity, irritability - mostly children). Very low acute toxicity Interactions: When administered with valproic acid, can induce non-convulsive (absence) status epilepticus (rare). D. Lorazepam (Ativan) or Diazepam (Valium) epilepsy in 1960’s) (benzodiazepines; Introduced for use in Mechanism: Same as clonazepam Uses: i.v. for Status epilepticus (lorazepam preferred because of longer duration of action) Toxicity: Same as clonazepam E. Ethosuximide (Zarontin; succinimide derivative; Introduced in early 1960’s) Mechanism: Blocks Ca++ T currents in thalamic neurons and reduces thalamocortical reverberating neuronal activity. Uses: Absence seizures (drug of choice) 10 F. Metabolism: 25% excreted unchanged; hydroxyethyl derivative is major hepatic metabolite (40%) and is inactive. t1/2 = 40 - 50 h. Toxicity: Gastrointestinal disturbances (nausea, vomiting, anorexia) and CNS effects (drowsiness, dizziness, euphoria, headache, hiccup). Interactions: None Phenobarbital (Luminol; barbituric acid derivative; Introduced in the U.S. in 1918) Mechanism: Enhances binding of GABA to receptors and increases the open-time of chloride channels in the presence of synaptically-released GABA. Uses: Still an effective antiseizure drug used for generalized tonic-clonic and partial seizures. G. H. Metabolism: Up to 25% excreted unchanged. hydroxyphenyl derivative (inactive). t1/2 = 100 h. Major hepatic metabolite is p- Toxicity: Sedation (partial tolerance develops) and altered behavior in children (irritability and hyperactivity) and the elderly (agitation and confusion).. Interactions: Induces hepatic drug metabolizing enzymes. Primidone (Mysoline; barbituric acid derivative; Introduced in late 1950’s) Mechanism: Same as phenobarbital Uses: Same as phenobarbital Metabolism: t1/2 = 5 - 15 h; converted to two active metabolites - phenobarbital and phenylethylmalonamide. 40% excreted unchanged.; Toxicity: Similar to phenobarbital, but can be more severe when therapy first started. Acute feeling of intoxication reported immediately after administration. Interactions: Induces hepatic drug metabolizing enzymes; phenytoin can increase conversion of primidone to phenobarbital. Valproic Acid (Depakene; n-dipropylacetic acid; approved in U.S. in 1978) Mechanism: Absence seizures: blocks Ca++ T currents in thalamic neurons. Tonic/clonic seizures: Slows reactivation of Na+ channels; ↑GABA by ↑synthesis and ↓ degradation Metabolism: Essentially complete; major metabolite = ester glucuronide; t1/2 = 15 h. Uses: Absence seizures, generalized and partial seizures, myoclonic seizures, and 11 atonic seizures. Toxicity: Very low acute toxicity. Common side effects: gastrointestinal symptoms (anorexia, nausea, and vomiting). Rare side effect is idiosyncratic hepatotoxicity (fulminant hepatitis). High doses may inhibit platelet function and produce bruising and gingival bleeding. Interactions: Inhibits hepatic metabolism of phenobarbital; High degree of plasma protein binding and may displace other drugs; Can cause absence status epilepticus when co-administered with clonazepam (rare). IX. “Second-Generation” Antiseizure Drugs in Clinical Use A. Gabapentin (Neurontin; FDA approved in 1993) Mechanism: Originally designed as a lipophilic GABA agonist, it does not interact with GABA receptors or affect reuptake or synthesis. However, it does increase the promoted release of GABA by an unknown mechanism. Uses: Primarily an add-on drug for control of partial and tonic-clonic seizures arising from partial seizures. Metabolism: none, excreted unchanged in urine. Toxicity: Interactions: None Very low. Few side effects; transient sedation, dizziness and GI upset. B. Pregabalin (Lyrica; FDA Approved in 2005). Mechanism: Designed based on gabapentin structure and has similar activity but is more potent. Mechanism of action unknown. Uses: Primarily an add-on drug for control of partial seizures with or without secondary generalization; also effective in treating neuropathic pain. Metabolism: none, excreted unchanged in urine. Toxicity: Very low. Few side effects; somnolence, dizziness most common. Interactions: None C. Lamotrigine (Lamictal; FDA approved in 1994) Mechanism: Slows rate of recovery of voltage-gated Na+ channels from inactivation, thereby prolonging the absolute and relative refractory periods of neurons. Uses: Broad spectrum of activity makes it useful as add-on or monotherapy in 12 partial and secondarily generalized tonic-clonic seizures. May also be useful in absence seizures. Metabolism: Metabolized primarily by glucuronidation. t1/2 = 24 h. t1/2 is reduced to 15 h in presence of phenytoin, carbamazepine, or barbiturates and increased to 50 h in presence of valproic acid. Toxicity: Dizziness, ataxia, blurred/double vision, GI upset, rash. Interactions: Rash more prevalent when given with valproic acid; concurrent use with carbamazepine increases 10,11-epoxide metabolite which can result in greater toxicity. D. Felbamate (Felbatol; FDA approved in 1993) Mechanism: Slows rate of recovery of voltage-gated Na+ channels from inactivation and has clear inhibitory effects on the excitatory NMDA receptor. Blocks seizures in both animal models of epilepsy. Uses: As add-on or monotherapy in partial and generalized seizures, but only for patients uncontrolled by other agents. Withdrawn from general use because of risk of aplastic anemia. Metabolism: Metabolized in liver. t1/2 = 20 h. Toxicity: GI upset, headache and insomnia at beginning of therapy. Weight loss and anorexia in prolonged use. Aplastic anemia (1/3,500 patients), hepatic failure (1/30,000 patients). Interactions: Inhibits metabolism of phenytoin, phenobarbital, valproic acid and carbamazepine 10,11-epoxide. E. Topiramate (Topamax; FDA approved in 1996) Mechanism: Slows rate of recovery of voltage-gated Na+ channels from inactivation; Enhances activity of GABA; Blocks action of glutamate. Uses: FDA approved as add-on therapy for partial seizures; may be of use in generalized tonicclonic seizures. Metabolism: 50 – 80% of dose is excreted by the kidney unchanged, with approximately 20% metabolized. t1/2 = 20 to 30 h. Toxicity: Dizziness, somnolence, ataxia, blurred/double vision, GI upset; increased risk of kidney stones due to inhibition of carbonic anhydrase. Interactions: Increases phenytoin concentration by 25%; Phenytoin co-administration decreases topiramate by 48% and carbamazepine decreases it by 40%. F. Fosphenytoin (Cerebyx; FDA approved in 1996). Mechanism: Same as phenytoin 13 Uses: Intravenous administration for treating status epilepticus. Is a water-soluble prodrug of phenytoin that can be safely administered i.v. or i.m. Eliminates the cardiac toxicity of i.v. phenytoin due to propylene glycol necessary to dissolve it. Can be infused 3 x faster than phenytoin. Metabolism: Converted to phenytoin in 8 - 15 min. t1/2 = 26 h. Toxicity: Same as phenytoin. Hypotension during i.v. administration. Interactions: See phenytoin F. Levetiracetam (Keppra; FDA approved in 1999). Mechanism: Mechanism of action unknown. Inhibits partial and secondarily generalized tonicclonic seizures in the kindling model, but not electroshock or pentylenetetrazol-induced seizures. Uses: Add on therapy for partial and secondarily generalized tonic-clonic seizures; monotherapy being investigated. Metabolism: 65% excreted unchanged; 25% metabolized by hydrolysis of acetamide. Not a substrate for cyt P450 nor does it induce enzymes. Toxicity: Generally well tolerated; most frequent side effects are somnolence, asthenia (muscle weakness), dizziness. Interactions: None known G. Tiagabine (Gabitril; FDA approved in 1998). Mechanism: Inhibits GAT-1, GABA transporter, thereby reducing re-uptake of GABA and prolonging inhibitory synaptic currents. Inhibits partial and secondarily generalized tonic-clonic seizures in the kindling model and maximum electroshock seizures. Uses: Add on therapy for partial and secondarily generalized tonic-clonic seizures; efficacy for monotherapy not established. Metabolism: Extensively bound to plasma protein; metabolized by CYP3A in the liver. Half life = 8 h, but only 2 –3 h when coadministered with enzyme inducing drugs. Toxicity: Most frequent side effects: mild to moderate somnolence, dizziness and tremor. Facilitates spike and wave discharge in animal models of absence seizures, thus may be contraindicated in patients with absence seizures. Interactions: None known H. Zonisamide (Zonegran; FDA approved in 2000). Mechanism: Inhibits T-type calcium currents; Prolongs inactivation state of sodium channels. Inhibits partial and secondarily generalized tonic-clonic seizures in the kindling model and 14 maximum electroshock seizures. Uses: Add on therapy for partial seizures; small studies suggest possible use in absence seizures and generalized tonic-clonic seizures. Metabolism: 40% bound to plasma protein; 85% excreted as a mixture of unchanged zonisamide and a metabolite of a CYP3A that is futher glucuronidated; doesn’t affect metabolism of other antiseizure drugs. Toxicity: Generally well tolerated; most frequent side effects are somnolence, ataxia, anorexia, nervousness and fatigue. Approx. 1 – 2.5% patients develop renal calculi. Interactions: None known I. Oxcarbazepine (Trileptal; FDA approved in 2000). Mechanism: See Carbamazepine; Is a keto- analog of carbamazepine that is immediately metabolized to active 10-monohydroxy derivative. Uses: Approved for monotherapy or adjunct therapy for partial seizures. Metabolism: Glucuronide conjugation and excretion. Less potent inducer of hepatic enzymes than carbamazepine but does induce CYP3A. Toxicity: Similar to carbamazepine, except better tolerated because of lack of metabolism to 10,11-epoxide which mediates some of carbamazepines toxic effects. Interactions: See carbamazepine 15 X. SUMMARY OF DRUGS USED FOR THERAPY Seizure Type Drug(s) of Choice Alternate Drugs Partial Seizures (including simple, complex and secondarily generalized tonic-clonic seizures) Carbamazepine Valproic Acid Phenytoin Phenobarbital Primidone Gabapentin* Lamotrigine* Levetiracetam Tiagabine Topiramate* Zonisamide Oxcarbazepine* Generalized TonicClonic (Grand Mal) Valproic Acid Phenytoin Carbamazepine Phenobarbital Primidone Lamotrigine Topiramate General Absence (petit mal) Ethosuximide Valproic Acid (first choice if tonic-clonic seizures present) Lamotrigine* Clonazepam (not first choice because of tolerance) Myoclonic Valproic Acid Clonazepam Lamotrigine Status Epilepticus Lorazepam (i.v.) Phenytoin or Fosphenytoin, (i.v.) (alone or in combination with diazepam) Diazepam (i.v.) *On the basis of an evidence-based review of literature and trials of their efficacy, these agents were recommended as potential drugs to be prescribed as monotherapy for newly diagnosed cases of epilepsy by subcommittees of the American Academy of Neurology and American Epilepsy Society (see French et al., Neurology, 62:1252-1260, 2004). Of these agents, only Oxcarbazepine is FDA approved for this purpose. 16