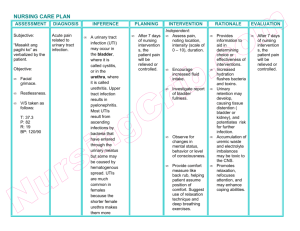
Pathogenesis Enters the body through the gastrointestinal tract after
advertisement
Pathogenesis Enters the body through the gastrointestinal tract after ingestion of contaminated foods, phagocytosis into the epithelial cells by interaction of internalin (A & B) protein with E cadherin (receptor on epithelial cells) enclosed in a phagolysosome where the low PH activates the bacteria to produce lysteriolysine O, this enzyme lyses the membrane & allows the listeriae to escape into the cytoplasm of the E.c, when they are proliferate on & induce host cell actin polymerization. Causes listeriosis (granulomatosis infant septica) may be an intrauterine infection. The last onset syndrome causes the development of the meningitis. 1) Actinomycetes: Large, diverse group of G +ve, bacilli with a tendency to form chains or filaments, facultative anaerobes that grow best in an atmosphere with increased CO2 , young colonies produce G +ve substrate filament that fragment into short chains. Species are identified based on cell wall chemotype & biochemical reactions. Pathogenesis & pathology The bacteria bridge the mucosal or epithelial surface of the mouth, respiratory tract, or lower gastrointestinal tract associated with dental caries, gin gin surgical complication or trauma. Asperation this bacteria may lead to pulmonary infection. The symptoms of thoratic Antinomycosis resemble those of a sub acute pulmonary infection; mild fever, cough & purulent sputum. Diagnostic laboratory tests Pus from draining sinuses, or specimens of tissue are examined for the presence of sulfur granules that are hard, lobulated & composed of tissue & bacterial filaments, which are club shaped at the periphery. Gram –ve rods (Enterobacteriaceae) :This family is a large, heterogenous group of G –ve, rods whose natural habitat is the intestinal tract of human & animals. Some enteric organisms e.g. Escherichia coli are part of the normal flora & incidentally cause disease, while others (salmonellae & shigellae ) are regularly pathogenic for humans. This family has the following characteristics : They are gram negative rods : Either motile with peritrichous flagella or non motile. They grow well on MacConkey agar. Grow aerobically & facultative anaerobes. Ferment glucose, often with gas production. Catalase positive. Oxidase negative. Reduce nitrate to nitrite. Growth characteristics & biochemical identification: 1) Carbohydrate fermentation 2) Aminoacid decarboxylase 3) Indole production from tryptophan 4) Voges-proskuer 5) Culture of differential media such as EMB & MacConkey agar. 6) Culture in TSI or KI 7) Culture in Christensen’s urea media for detective of urease production. Antigenic structure: 1) Enterobacteriaceae have a complex antigenic structure such as : 1. O antigens represented by LPS. Some O-specific polysaccharides contain unique sugars, but others are different. 2. K antigens found in some members of this family. E. coli producing K antigen cause attach of the bacteria to epithelial cells of GIT or UT, but klebsiella form large capsules consisting of polysaccharides (k antigen). 3. H antigens: are located on flagella the determinants in H antigens are a function of the amino acid sequence in flagellar protein (flagellin). 2) Colicins (Bacteriocins) : These virus-like bactericidal substances are produced by certain strains of bacteria active against some other strains of the same or closely related species.The Bacteriocin-producing strains are resistant to their own bacteriocin, thus it can be used for “typing” of organisms. 3) Toxin & Enzymes: LPS (endotoxin), exotoxin & many enzymes. Escherichia : E. coli is motile & may produce polysaccharide capsule. Most strains ferment lactose. Producing small red colonies on MacConkey agar. Certain strains are haemolytic when grown on media containing suitable erythrocytes. Antigenic structure: LPS or somatic O antigens Flagellar H antigens Capsular K antigens Fimbrial antigens : type 1 fimbriae can mediate adhesion on a wide range of human & animal cells that contain the sugar mannose. Pathogenesis Strains of E. coli possess a range of different pathogenic mechanisms. The polysaccharides of the O & K antigens protect the organism from the bacteriocidal effect of complement & phagocytes in the absence of specific antibodies. Clinical syndromes Urinary tract & septic infections The infections thought to occur in ascending manner for physiological reasons. There is evidence that the ability of E. coli to infect the urinary tract is associated with fimbriae that specifically mediate adherence to the Uro-epithelial cells. Diarrhea E. coli may cause gastro-intestinal disease ranging in severity from mild, self-limiting diarrhea to haemorrhagic colitis. Such strains fall into at least 5 groups: 1) Enteropathogenic E. coli (EPEC): 2) Enterotoxigenic E. coli (ETEC): 3) Enteroinvasive E. coli (EIEC): 4) Verocytotoxin- producing E. coli (VTEC): 5) Enteroaggregative E. coli (EAggEC): Klebsiella : Klebsiella have capsular material which is produced in greater amounts on media rich in carbohydrate. The capsule are all complex acid polysaccharides, contain glucuronic acid & pyruvic acid. They resemble the K antigens of E. coli. There is no haemolysis of horse or sheep red cells and no motile. Antigenic structure: Capsular K antigens Capsular O antigens Bacteriocins: distinct from colicins because they have no action onE. coli. Pathogenesis K. pneumonia is a fairly common cause of UTI & occasionally give rise to cases of sever bronchopneumonia, some times with chronic destructive lesions & multiple abscess formation in the lungs, in many can reach to blood and causes bacteremia. Enterobacter : Have many features in common with those of the klebsiella; but distinguished by their motility, the colonies may be slightly mucoid, express a lysine decarboxylase enzyme but not arginine decarboxylase. Enterobacter cloacae, Enterobacter aerogenes are the most important clinically. The normal habitat is soil & water & is occasionally formed in human feces & in the R.T. Antigenic structure & virulence factors: Type I & type III fimbriae Most strains also express an aerobactein- mediated iron uptake system. α- haemolysin Ompx (outer membrane protein) Pathogenesis The pathogenic mechanisms of these bacteria are poorly understood, but Ompx (outer membrane) may be a pathogenic factor for strains of Ent. cloacae, this protein appears to reduce production of porins, leading to decreased sensitivity to βlactam antibiotics, & might play a role in host cell invasion. Serratia : The S. marcescens is the most commonly encountered in clinical specimens. Capsules are not normally formed but capsular material is formed on a well aerated medium, poor in nitrogen & phosphate. Most Serratia strains are motile & some strains of S. marcescens produce red pigmented colonies on agar. The pigment is formed only in the presence of oxygen & at a suitable temperature; at lower temperatures growth is poorer & pigment formation is abundant. Pathogenesis Most infections occur in hospital patients; they include wound infection, septicemia, & Endocardiatis. Extracellular enzymes may be responsible for host tissue damage. Toxins resembling E. coli verocytotoxin & heat-labile toxin have been described. Proteus : There is considerable morphological variation, but in agar grown culture the microscopical appearance is much like that of the other coliform bacteria. All grow well on laboratory nutrient media. A notable property of Pr. vulgaris & Pr. mirabilis is the ability to swarm on solid media; the bacterial growth spreads progressively from the edge of the colony & eventually covers the whole surface of the medium, & this growth takes place in discontinuous manner, with each period of outward with progress followed by a stationary period. Tests Indole Citrate H2S Urease Lipase Gelalinase Aesculin. H. Swarming Fermentation Manose Maltose Xylose Salicin p. mirabilis v + + + + + P. vulgaris + + + v + + + P. morgani + + - + - + + + + - Pathogenesis These bacteria create alkaline conditions in the urine because of the ability to produce urease enzyme. These bacteria may provoke the formation of calculi (stones) in the urinary tract. Shigellae : The natural habitat of Shigellae is limited to the intestinal tracts of human & other primates, where they produce bacillary dysentery. G-ve bacilli, non motile, non capsulated, Shigellae are facultative anaerobes but grow best aerobically, convex, circular, transparent colonies with intact edges, ferment glucose, not ferment lactose. The genus is subdivided on biochemical & serological grands into four species : Sh. flexneri Sh. boydii Sh .sonnei Sh. dysenteriae Antigenic structure: Have (O) antigens (LPS), which have a complex antigenic pattern. Toxins Endotoxin (probably contributes to the irritation of the bowel wall) Shigell dysenteriae Exotoxin (heat labile exotoxin that affects on both the gut & the central nervous system). It is protein acting as an enterotoxin it produces diarrhea similar to E. coli verotoxin. Pathogenesis Infection limited to the gastrointestinal tract; blood stream invasion is quite rare. The infective dose is in the order of 10 3 organisms (whereas it usually is 105 - 108 for Salmonella & Vibrios). Essential pathologic process include: Invasion of mucosal epithelial cells (M cell) by induced phagocytosis. Escape from the phagocytic vacuole, multiplication & spread within the epithelial cell cytoplasm & passage to adjacent cells. Microabscesses in the wall of the large intestine & terminal ileum lead to necrosis of the mucous membrane, superficial ulceration, bleeding & formation of a “Pseudomembrane” on the ulcerated area, (this consists of fibrin, leukocytes, cell debris, a necrotic mucous membrane & bacteria). Granulation tissue fills the ulcers & scar tissue forms. Diagnostic laboratory tests Specimens: fresh stool, mucus flecks & rectal swabs for culture. Large numbers of fecal leukocytes & some red blood cells often are seen microscopically. Culture: grow on differential media (MacConkey & EMB) & on selective media (Hektoen enteric agar or S.S agar), appear colorless lactose negative colonies. On TsI fail to produce H2S with acid but not gas in the bottom & an alkaline slant. Serology is not used to diagnose Shigella infections. Salmonella : Salmonella are often pathogenic for humans or animals when acquired by the oral route. Motile with peritrichous flagella, they never ferment lactose or sucrose, produce H2S. Classification of Salmonellae is based on biochemical reaction & structures of the O, H & V antigens. There are many serotypes: S. typhi S. paratyphi A, B S. choleraesuis S. typhimurium S. enteritidis Pathogenesis & clinical findings The organisms almost always enter via the oral route, usually with contaminated food or drink.The host factors that contribute to resistance to Salmonella infection are: Gastric acidity Normal intestinal microbial flora Local intestinal immunity. Salmonellae produce three main types of disease in humans: A) Enteric fevers(Typhoid fever) Produced by only a few of the Salmonellae (Salmonella typhi), the ingested salmonellae reach the small intestine, from which they enter the lymphatic & then the bloodstream; they are carried by the blood to many organs including the intestine. The organisms multiply in intestinal lymphoid tissue & are excreted in stools. After an incubation period of 10-14 days, fever, malaise, headache, constipation, bradycardia & myalgia occur. The fever rises to a high plateau & the spleen & liver become enlarged. Rose spots, usually on the skin of the abdomen or chest are seen. The chief complications of enteric fever were intestinal hemorrhage & perforation. B) Bacteremia focal lesions This is associated commonly with Salmonella choleraesuis but may be caused by any Salmonella serotype. Following oral infection, there is early invasion of the blood stream with focal lesions in lungs, bone, meninges, but intestinal manifestations are often absent. Blood cultures are positive. Enterocolitis : This is the most common manifestation of Salmonella infection ex: S. typhimurin & S. enteritidis. After 48 hr of ingestion of bacteria (the bacteria multiply & invade the intestinal mucosa), there is nausea, headache, vomiting & profuse diarrhea. Blood cultures are usually negative, but stool cultures are positive. Diagnostic laboratory tests 1. Specimens : Blood culture in enteric fevers are often positive in the first week of infection, urine cultures may be positive after the second week & stool culture yield positive from the second or third week, but for the enterocolitis the stool culture is positive during the first week. 2. Bacteriological methods for isolation of Salmonellae : a) Differential medium culture: EMB-Macc-Deoxycholate medium permits rapid detection of lactose non fermenters (S.shi-pro-ser-pseu….) & G+ve are inhibited. Bismuth sulfite medium permits rapid detection of S. typhi, which forms black colonies of H2S production. b) Selective media: S.S agar, deoxycholate citrate agar (XLD). Favor growth of Salmonellae & Shigella. Salmonella-Shigella agar plate (SS c) Enrichment culture: Tetrathionate broth inhibits replication of normal intestinal bacteria & permit multiplication of Salmonellae. d) Biochemical identification: by using many biochemical reactions for detection & identification of suspect colonies. 3. Serologic methods: identify unknown cultures with known sera. These include: Agglutination tests: the known sera & unknown culture are mixed on a slide, clumping occurs within a few minutes. There are commercial kits available to agglutinate & serogroup salmonellae by their O antigens. Tube dilution agglutination tests (Widal test): the serum agglutinins rise sharply during the second & third weeks of salmonellae infection. Serial twofold dilutions of unknown serum are tested against antigens from representative Salmonella. Results are interpreted as follows: 1) High or rising titer of O (≥1:160) suggests that active infection is present. 2) High titer of H (≥1:160) suggests past immunization or past infection. 3) High titer of antibody to the V antigen occurs in some carriers. Other G-ve bacteria :- Pseudomonas aeruginosa : Is G –ve, motile, aerobic rods some of which produce watersoluble pigments, occurs as single bacteria, in pairs & occasionally in short chains. Widely distributed in nature & is commonly present in moist environments in hospitals. It causes disease in humans with abnormal host defense. Grow readily on many types of culture media, produces smooth round colonies with fluorescent bluish pigment Pyocyanin, which diffuses into the agar, sometimes producing a sweet or grape-like or corntaco-like odor. Some strains hemolyze blood, & many strains also give a greenish color to the agar because of the ability to produce fluorescent pigment pyoverdin, but other strains give black pigment pyomelanin. It grows well at 37-42 °C, oxidase positive. It does not ferment carbohydrate but oxidize glucose. The growth at 42 °C, with presence of characteristic pigments is important in differentiation. Antigenic structure (Virulence factor) & enzyme, toxin Pili Capsules (responsible for the mucoid colonies) LPS Elastases, proteases (play key role in corneal ulceration) & two hemolysins: Heat labile phospholipase C Heat stable glycolipid Exotoxin (A) blocks protein synthesis by mechanism similar to Diphtheria toxin. Phospholipases, proteases & alginate chronic pulmonary colonization. associated with Fluorecein or pyoverdin pigments act as bacterial siderophores. Pathogenesis P. aeruginosa is pathogen when introduced into: Areas devoid of normal defense ex: mucous membrane & skin are disrupted When intravenous or urinary catheters are used When neutropenia is present as in cancer chemotherapy. The pathogen process The bacteria attaches to & colonizes the mucous membranes or skin, invade locally & produces systemic disease, these processes are promoted by the pili, enzymes & toxins but LPS plays direct role in causing fever, shock, Oliguria, leukocytosis & leukopenia & adult respiratory distress syndrome. Diagnostic laboratory tests Specimens: Pus, urine, blood, spinal fluid & sputum Culture: Grow on blood agar & on differential media used to grow the enteric G –ve rods. It is not lactose ferment. Vibrios : V. cholerae transmission in water & is comma shaped, curved rod, actively motile by polar flagellum, produce convex, smooth round colonies, grow well on thiosulfate – citrate bile sucrose (TCBS) agar, on which it produces yellow colonies, able to grow at a very high PH (8.5 – 9.5) & are rapidly killed by acid. Most Vibrio species are halotolerant & Nacl often stimulates their growth. Antigenic structure : Heat – labile flagellar H antigen O LPS that confer serologic specificity. There are at least 139 O antigen groups. Toxin V. cholerae produce heat – labile enterotoxin, consisting of subunits A&B. The somatic ganglioside GM1 in human & mammalian cell serves as the mucosal receptor for subunit B, which promotes entry of subunit A, into the cell. The activation of subunit A yields increased levels of intra cellular CAMP & results in prolonged hypersecretion of water & electrolytes. There is increased sodium dependent chloride secretion, & absorption of sodium & chloride is inhibited. Diarrhea occurs with resulting dehydration, shock, acidosis & death. Pathology Is pathogenic only for humans. Any medication or condition that decreases stomach acidity makes a person more susceptible to infection with V. cholerae. Cholera is not an invasive infect, the organism do not reach the blood stream but remain within the intestinal tract. The V. cholerae attach to the microvilli of the brush border of epithelial cells. There they multiply & liberate cholera toxin & perhaps mucinasses & endotoxin. Diagnostic laboratory tests Specimens: stools Smears: dark – field or phase contrast microscopy may show the rapidly motile vibrios. Culture: growth is rapid in peptone agar & on blood agar with PH near 9 or on TCBs agar. A few drops of stool can be incubated for 6 – 8 hrs in taurocholate – peptone broth (PH: 8 – 9) the organisms from this culture can be stained or subcultured. Specific tests: V. cholerae organisms are furthure identified by slide agglutination tests using anti O group. O1 & O139 antiserum & by biochemical reaction pattern. Campylobacter : Cause both diarrheal & systemic disease. The classification of bacteria within the family Campylobacteriaceae has change frequently; There are 2 species: C. jejuni C. fetus C. jejuni Are common human pathogens, causing mainly enteritis & occasionally systemic infection. G –ve, rod with comma S, or “gull – wing“ shapes, motile with single polar flagellum. The colonies tend to be colorless or grey, they may be watery & spreading or round & convex & both colony types may appear on one agar plate such as Skirraus medium contain vancomycin, polymyxin & trimethoprim. Oxidase +ve, catalase +ve, non oxidase or ferment carbohydrate, nitrate reduction & H2S production. Antigenic structure & biologic: LPS Cytopathic extra cellular toxins Enterotoxin Pathogenesis & Pathology Acquired by the oral route (food, drink) or contact with infected animals or animal products. 104 organisms are necessary to produce infection. Organisms multiply In the small intestine, invade the epithelium & produce inflammation that results in the appearance of red & white blood cells in the stools, occasionally, the bloodstream is invaded & clinical picture of enteric fever develops. Campylobacter fetus Is opportunistic pathogen that causes systemic infection in Immune compromised patients. It may occasionally cause diarrhea. The gastrointestinal tract may be the portal of entry. Have surface array proteins which form a capsule like structure of the organism. Helicobacter pylori : Is a spiral – shaped G –ve rod, is associated with natural gastritis, duodenal (peptic) ulcer disease, gastric ulcers & gastric carcinoma. Have many characteristics in common with Campylobacter. It has multiple flagella at one pole & is actively motile. The media for primary isolation include Skirrows medium with vancomycin, polymyxin B & trimethoprim, chocolate medium. The colonies are translucent, oxidase +ve, catalase –ve, strong producer of urease. Pathogenesis On the lumen side of the mucus, the PH is low (1 – 2), while on the epithelial side the PH is about (7.4). H. pylori is found deep in the mucus layer, near the epithelial surface where physiologic PH is present, produce protease that modifies the gastric mucus & further reduces the ability of acid to diffuse through the mucus. Ingestion of H. pylori resulted in development of gastritis & hypochlorhydria (which occurs when the stomach produces insufficient amount of hydrochloric acid. This condition is usually mistaken for acidity because the hydrochloric acid is : needed for the breakdown for protein in the stomach. To help with the absorption of nutrients such as calcium & iron. To control the growth of unwanted unicroorganisms in the digestive tract.) & found associated between the presence of bacteria infection & duodenal ulceration. The mechanisms by which H. pylori causes mucosal inflamation & damage are not well defined but probably involve both bacterial & host factors. Bacteria invade epithelial cell damage mucosal cells H. pylori surface to a limited degree also by ammonia Toxin & LPS which damage produced by the urease activity Chronic & active inflammation (gastritis) Diagnostic laboratory Specimens: gastric biopsy specimen Antibodies: several assays have been developed to detect serum Ab, specific for H. pylori. Heamophilus influenzae : It is found on the mucous membranes of the upper respiratory tract in humans. It is a cause of meningitis in children & occasionally causes of respiratory tract infection in children & adults. G –ve, cocco bacilli, sometimes occurs in pairs or short chains (pleomorphic forms). They have definite capsule. Small, round, convex colonies appear on BHIA with blood, is not hemolytic. Around staphylococcal colonies these bacteria grow much larger (satellite phenomena). Need certain growth factors called X & V. Factor X acts physiologically as hemin; factor V can be replaced by nicotinamide adenine dinucleotide (NAD) or other coenzymes. Carbohydrates are fermented poorly. Antigenic structure: Capsule LPS Outer membrane proteins Pathogenesis The non encapsulated organ is a regular member of the normal respiratory flora of humans. The polyribose phosphate capsule of type B H. influenzae is the major virulence factor, therefore is responsible for meningitis & pneumonia. Bacteria type b extension with enters through the R.T local involvement of the sinuses or the middle ear. Less frequency, may establish themselves in the joints to produce septic arthritis reach the blood stream & may be carried to the membrane Bordetellae : B. pertussis, a highly communicable & important pathogen for humans, G –ve coccobacilli resembling H. influenzae with toluidine blue stain, bipolar metachromatic granules can be demonstrated. Capsule is present, strict aerobe & forms acid but not gas from glucose & lactose. It does not require X & V factors on subculture. Hemolysis of blood-containing medium is associated with virulent B. pertussis. Bordet-Gengou medium (potatoblood-glycerol agar). The organism structures: has many antigenic Pili Pertussis toxin Adenylyl cyclase toxin Dermonecrotic toxin Hemolysin Tracheal cytotoxin These bacteria survive for only brief periods outside the human host. There are no vectors; transmission is largely by the respiratory route. Bacteria adheres & multiplies rapidly Liberate the Bacteria adheres & multiplies toxins on the epithelial surface of onrapidly the epithelial surface of the trachea the trachea & bronchi & interfere that with ciliary action Liberate the toxins & substances that irritate & substances surface cells, causing coughing & marked lymphocytosis & bronchi & interfere with ciliary action cells, irritate surface causing coughing & marked lymphocytosis Obstruction of the smaller bronchioles by mucous plugs Obstruction of the smaller bronchioles by mucous infiltration withplugs There may be necrosis o parts of the epithelium & There may be necrosis of parts of polymorph nuclear (PMN)& polymorph the epithelium nuclear (PMN) & infiltration with peribronchial inflammation peribronchial inflammation & intensive pneumonia Contributes to the frequency of convulsions in infant Contributes to the frequency withofwhooping cough convulsions in infant with intensive pneumonia whooping cough Diagnostic laboratory tests Specimens: Saline nasal wash, Nasopharyngeal swabs or cough droplets expelled onto a Cough plate held in front of the patient’s mouth. Direct fluorescent Ab test: can be used to examine nasopharyngeal swab specimens, however false positive & negative results may occur, is most useful in identifying B. pertusis after culture on solid media. Culture: The saline nasal wash fluid is cultured on solid medium agar, the M.O are identified by IFS or by slide agglutination with specific antiserum. Brucella : Obligate parasites of animals & humans, are located intracellularly. 1) B. melitensis infects goats 2) B. suis 3) B. abortus 4) B. conis infects swine infects cattle infects dogs The bacteria varies from cocci to rods in length, G –ve, aerobic & non - motile, form small, convex, smooth colonies. Their nutritional requirements are complex & cultivated on defined media containing amino acids, vitamins, salts & glucose. It utilizes carbohydrates but produce neither acid nor gas, catalase positive, oxidase positive, H2S produced by many strains & Nitrates are reduced to nitrites. Antigenic structure: 2 Lipopolysaccharide antigen, A & M Superficial L antigen (resembles the V antigen of Salmonella) Pathogenesis The common routes of infection in human are the intestinal tract, mucosal membrane (droplets) & skin (contact with infected tissues of animal). The organism progress from the portal of entry, via lymphatic channels & regional lymph nodes, to the thoratic duct & the blood stream, which distributes them to the parenchymatous organs. Granulomatous nodules that may develop into abscesses form in lymphatic tissue, liver, spleen, bone marrow, & other parts of the reticuloendothelial system. Osteomyelidis, meningitis & cholecystitis also occasionally occurs. The incubation period is 1-6 weeks. The onset is insidious, with malaise, fever, weakness, aches & sweats. Diagnostic laboratory tests Specimens: Blood, biopsy material & serum for serological tests. Culture: Specimens are incubated in trypticase-soy broth & on thionine-tryptose agar. Sub cultured for at least 3 weeks before being reported as negative. Serology: The IgM levels rise during the first week of acute illness, peak at 3 months & may persist during chronic disease. Agglutination test: must be performed with standarized heat killed, phenolized, smooth brucella antigens. IgG agglutination liters above 1:80 indicate active infection. If the serum agglutination test is negative in patients with strong clinical evidence of brucella infections; tests must be made for the presence of blocking antibodies. Blocking antibodies: These are IgA antibodies that interfere with agglutination by IgG & IgM can cause a serologic test to be negative in low serum dilutions (prozone) although positive in higher dilutions. Yersinia : The genus Yersinia includes Yersinia pestis, the cause of plague. Yersinia pseudotuberculosis & Y. enterocolitica are important causes of human diarrheal diseases. Y. pestis is G –ve rod that exhibits striking bipolar staining with special stains, non-motile. Growth is more rapid in media containing blood or tissue fluids; in blood agar colonies maybe very small at 24 hr & when derived from infected tissue produce gray & viscous colonies but after passage to the laboratory the colonies become irregular & rough. The organism has little biochemical activity. Antigenic structure: LPS Exotoxin Envelope contains a protein (fraction I) acts as antiphagocytic properties. A 72-kb plasmid is essential for virulence Coagulase Bacteriocin (pesticin) Isocitrate lyase Pathogenesis Flea feeds on a rodent infected with Y. pestis Ingested M.O multiplies in the gut of the flea & helped by the coagulase, block its proventriculus so that no food can pass through. Blocked & hungry flea bites ferociously & the aspirated blood, contaminated with Y. pestis from the flea, is regurgitated into the bite wound. The M.O may be phagocytosed by PMN cells & monocytes. The Y. pestis are killed by the PMN but multiply in the monocytes, because the bacteria are multiplying at 37°C they produce anti phagocytic proteins & subsequently able to resist phagocytosis. The pathogen rapidly reaches the lymphatics & intense hemorrhagic inflammation develops in the enlarged lymph nodes that undergo necrosis & become fluctuant; while the invasion may stop there, Y. pestis often reach the blood stream & become widely disseminated. Hemorrhagic & necrotic lesions may develop in all organs, meningitis, pneumonia & serosanguineous, pleuropericarditis are prominent features. The disease was known as a black death, because one of its characteristics is blackish areas on the skin covered by subcutaneous hemorrhages. Incubation period of 2–7 days, after it high fever& pain full lymphadenopathy, commonly with greatly enlarged, tender nodes (buboes) in the groin or axillae. Vomiting & diarrhea may develop with early sepsis, hypotension, renal & cardiac failure. Terminally, signs of pneumonia & meningitis can appear. Diagnostic laboratory tests a- Specimens: blood, sputum, CSF. b- Staining with Giemsa’s stain & with specific immunofluoresent stains. c- Culture: on blood agar & MacConkeys agar. d- Serology: a convalescent serum, Ab titer of 1:16 or greater is presumptive evidence of Y. pestis infection. A titer rise in two sequential speciment continues the serologic diagnosis. Pasteurella : Non motile, G –ve, cocco bacilli with a bipolar appearance on stained smears. Aerobes grow readily on ordinary bacteriologic media at 37°C, oxidase +ve, catalase +ve but diverge in other biochemical reactions. Many Pasteurella species are primarily are animal pathogens. Pasteurella multocida, P. haemolytica, P. pneumotropica. Clinical findings: The most common presentation is a history of animal bite followed within hours by an acute onset of redness, swelling & pain. Regional lymphadenopathy is variable & fever is often low – grade. Pasteurella infections sometimes present as bacteremia or chronic respiratory infection without an evident connection with animals. Mycobacteria tuberculosis : Tubercle bacilli are thin straight rods. It can not be classified as either G +ve or G –ve. Once stained by basic dyes, they can not be decolorized by alcohol, regardless of treatment with iodine. True tubercle bacilli are characterized by acid fastness, 95% ethyl alcohol containing 3% hydrochloric acid, quickly decolorize all bacteria except the mycobacteria. Acid fastness depends on the integrity of the waxy envelope. The mycobacteria can be demonstrated by yellow – orange fluorescence after staining with fluorescence stains. Culture : Bacteria grow on selective & non selective media. The selective medium contains Antibiotics to prevent the overgrowth of contaminated bacteria & fungi such as Lowenstein Jensen media which contain defined salts, glycerol & complex organic substances. Growth characteristics : Obligate aerobes & increased CO2 tension, enhances growth. Biochemical activities are not characteristic & the growth rate is much slower than that of most bacteria. The doubling time of these bacteria is about 18 hours, are resistant to drying & survives for long periods in dried sputum. These bacteria have variation in colony appearance, pigmentation, virulence, optimal growth temperature & many other cellular or growth characteristics. These bacteria contain many constituents in cell walls that can induce delayed hypersensitivity such as: Lipids (mycolic acids, waxes & phosphatides) Proteins bound to a wax fraction, can induce tuberculin & formation of a variety of Ab. Polysaccharides important in inducing the immediate type of hypersensitivity & act as antigens. Clinical findings: The tubercle can involve every organ system, its clinical manifestations are protean, fatigue, weakness, weight loss & fever may be signs of tuberculous disease. Pulmonary involvement giving rise to chronic cough & spitting of blood usually is associated with far advanced lesions. Meningitis or urinary tract involvement can occur in the absence of other signs of tuberculosis. Pathology: A) Two principle lesions: Productive type 1. Exudative type 2. 1. Exudative type: this consists of an acute inflammatory reaction with odema fluid, polymorphnuclear leukocytes and later, monocytes around the tubercle bacilli, this type is seen particularly in lung tissue. 2. Productive type: when fully developed, this lesion, achronic granuloma, consists of three zones : Central area of large multinucleated giant cell containing tubercle bacilli. Amid zone of pale epithelioid cell. A peripheral zone of fibroblasts, lymphocytes & monocytes. B) spread of the organism in the host C) intracellular site of growth Tubercle bacilli spread in the host by direct extension, though the lymphatic channels & blood stream & via bronchial & gastrointestinal tract. Once mycobacteria establish themselves in tissues, they reside principally intracellularly in monocytes, reticuloendothelium cells & giant cells. Lab diagnosis: Specimens: fresh sputum, gastric washings, urine, pleural fluid, cerebrospinal fluid…etc. The specimens are processed concentrated) before use. (decontaminated & Smear: Ziehl – neelson staining (sputum & exudates). Culture: selective media is Lowenstein – Jenson medium. Tuberclin test: Material: old tuberculin is concentrated filtrate of growth in which tubercle bacilli have grown for 6 weeks. PPD: Purified Protein Derivative is obtained by chemical fractionation of old tuberculin. Reaction to tuberculin: Skin test (Skin injection) “No reaction” An individual who has not had contact with mycobacteria develops indurations, edema, erythema in 24 – 48 hrs & with very intense reaction even central necrosis. An individual who has had a primary infection with TB. Mycobacterium leprae (causative agent of leprosy) : typical acid fast bacilli are found in scrapings from skin or mucous membranes in lepromatous leprosy. The organisms are often found with in the endothelial cells of blood vessels or mononuclear cells. Not grown in artificial media. The skin lesions may occur as pale, anesthetic macular lesionss1 – 10 cm. diffuse Infiltrate nodule (the erythematous) 1 – 5 cm. or diffuse (skin infiltration) Neurologic disturbance are manifested by nerve infiltration & thickening with anesthesia, neuritis, trophic ulcers & born resorption & shortening of digits. Two major type of leprosy: 1. lepromatous l. 2. tuberculoid l. Lab diagnosis: Specimens: scrapings from skin & nasal mucosa. Smeared on slide & stained by acid fast stain. There is no serological test of important. Treponema pallidium : Slender spiral, actively motile, rotating steadily around their endoflagella, even after attaching to cells by their tapered ends. Culture on artificial media, infertile eggs; it survives best in oxygen. Remain motile for 3 – 6 days at 25°C. Drying kills the spirochete rapidly as does elevation of the temperature to 42°C. Antigenic structure: T. pallidium can not be cultured in vitro, therefore limits the characterization of its antigens. The outer membrane surrounds the periplasmic space & the peptidoglycan – cytoplasmic membrane complex. Not contain lipopolysaccharide. Endo flagella. More than 100 protein antigens have been noted. Pathogenesis, pathology: A) Acquired syphilis: the infection with T. pallidium is limited to the human host & transmitted by sexual contact & the infectious lesion is on the skin or mucous membranes of genitalia. T. pallidium can penetrate intact mucous membranes or it may enter through a break in the epidermis. Multiply locally of the sit of entry. Spread to nearby lymph nodes & then reach the blood stream. In 2 – 10 weeks after infection, a papule develops of the site of infection & breaks down to form an ulcer. The inflammation is characterized by predominance of lymphocytes & plasma cells. (Primary lesion) heals spontaneously. After 2 – 10 weeks of primary lesion, the secondary lesions appear, these consist of a red maculopapular rash anywhere on the body. B) Congential syphilis: a pregnant syphilitic woman can transmit T. pallidium to the fetus through the placenta beginning in the 10th to 15th weeks of gestation. Diagnostic laboratory tests: Specimen: tissue fluid expressed from early surface lesions. Dark field examination: a drop of tissue fluid or exudates is placed on a slide & a cover slip pressed over it to make a thin layer. Show typical motile spirochetes under oil immersion. Immunofluorescence test: fluid spread on glass slide, air dried & fixed, stained with fluorescein labeled antitreponemal serum & examined by means of immunofluorescence microscope. Mycoplasmas : The mycolplasmas evolved from G +ve ancestors by reducing of genome size & are the smallest organisms that can be free living in nature & self replicating on laboratory media. They have the following characteristics: 1) The smallest size 2) Highly pleomorphic becomes, they lack a rigid cell wall & instead are bounded by a triple – layered “unit membrane” that contains a sterol. 3) They resist to penicillin because 4) They can reproduce in cell free media, on media 5) Have an affinity for mammalian cell membrane. Typical organisms: The morphology appears different according to the method of examination; can not be studied by the usual bacteriologic methods because of the small size of their colonies. Grow in heart infusion peptone broth with 20% agar PH (7.8) & 30% animal serum. These bacteria pass through filters with 450 nm pore size, non motile, many antigenically distinct species of mycoplasma have been isolated from animal & human; three species which are pathogen to human are: Ureplasma urealytium, M. hominis & M. pneumonia. Laboratory diagnostic tests: Specimens: throat swabs, sputum, exudates & genital secretion. Microscopic examination: direct examination is useless. Culture: specimen inoculated onto special solid media & incubation for 3-10 days at 37°C with 5% CO2. Serology: used CF tests & indirect immuno fluorescence. Pathogenecity include Genitourinary disease : U. urealytium, M. hominis are common parasites of the genital tract & their transmission is related to sexual activity, both of them can cause inflammation of the reproductive organs of male & female, because it is difficult to cultivate. The Mycoplasma treatment depends on recognition of clinical syndromes. Primary atypical pneumonia: has a bacterial origin. If bacterium can not be isolate the pneumonia is called atypical & a virus is usually suspected, if the virus can not be detected, then mycoplasmal pneumonia, can be considered this pneumonia caused by Mycoplasma pneumonia & they spread through close contact & some time air born droplet; the disease is fairly common & mild in infants & small children & young adults. The M. pneumonia usually infects the upper respiratory tract & move to the lower respiratory tract, where it attaches to respiratory mucosal cells. It then produces peroxide, which may be a toxin factor, but the exact mechanism of pathogenesis is unknown. This disease is ringed in severity from asymptomatic to a serous pneumonia. Initial symptoms include headache, weakness, a low fever, & a predominant cough. The subsequent events in infection are less well understood but may include several factors as follows: direct cytotoxicity through generation of hydrogen peroxide & superoxide radicals; cytolysis mediated by antigen antibody reactions or by chemotaxis & action of mononuclear cells & competition for & depletion of nutrients. Rickettsiae : Rickettsiae are pleomorphic coccobacilli, appearing either short rods or as cocci, they do not stain well with gram’s stain but are visible readily under the light microscope when they are stained with Giemsa. Grow readily in egg sacs of embryonated eggs. Also grow in cell culture & the generation time is 8-10 hr at 34 °C in this culture. These bacteria have G –ve cell wall structures & the Typhus & spotted fever groups contain LPS. These bacteria grow in different parts of the cells. In general Rickettsiae survive only for short times outside the vector or host, quickly destroyed by heat, drying & bactericidal chemicals. The organism may survive pasteurization at 60°C for 30 minutes & can survive for months in dried feces or milk. This may be due to the formation of endospore, like structures by Coxiella burnetii. They are obligate intracellular. Pathology: Rickettsiae except for C. burnetii multiply in endothelial cells of small blood vessels & produced vasculitis, the cell become swollen & necrotic & there is thrombosis of the vessel leading to rupture & necrosis. Clinical findings: Except for Q fever, in which there is no skin lesion Rickettsial infections are characterized by fever, headache, malaise, prostration, skin rash & enlargement of the spleen & liver. a- Typhus group Epidemic typhus Endemic typhus b- Spotted fever group c- Scrub typhus d- Q fever Laboratory findings: Isolation of Rickettsiae is technically different & is of only limited usefulness in diagnosis. The most widely used serologic tests are indirect immunofluorescence & complement fixation test. An antibody rise should be demonstrated during the course of the illness & diagnosis by symptoms & by Weil – felix reaction. Epidemic typhus: Is caused by R. prowazekii which is transmitted from person to person by the body louse; when a louse on an infected person. The Rickettsia infects the insect’s gut & multiplies & large numbers of organisms appear in the feces in about a week. When a louse takes a blood meal, it defecates the irritation causes the effected person to scratch the site & contaminate the bite wound with Rickettsia. The Rickettsia then spread via the blood stream & infect the endothelial cells of the blood vessels causing vasculitis (inflation of the blood vessel). Chlamydiae : Chlamydiae have distinctive staining properties (similar to those of Rickettsiae); the gram reaction of Chlamydiae is negative or variable & is not useful in identification of the agents. They are G –ve obligate intracellular parasites & very small in their size. Two species that cause human diseases are: Chlamydia trachomatis & Ch. psittaci. Some of the diseases are the following: 1. Inclusion conjunctivitis It is an acute infectious disease caused by Ch. trachomatis serotype D – K & it occurs throughout the world. It is characterized by a copious mucous discharge from the eye, an inflamed & swollen conjunctiva & the presence of large inclusion bodies. The infection newborn acquired the Chlamydiae during passage through an infected birth canal & the diseases appear 7–12 days after birth. The disease in adults acquired by contact with infective genital tract discharges, so the genital Chlamydial infections & inclusions conjunctiva are sexually transmitted diseases that are spread by indiscriminate contact with multiple sex partners. Growth & metabolism Chlamydiae require an intracellular habitat, because they are unable to synthesize ATP & depend on host cell for energy requirements. Chlamydiae grow in cultures of a variety of eukaryotic cell lines. Classification Are arranged according to their pathogenic potential: A) C. trachomatis B) C. pneumonia C) C. psttaci