Example 2 - Faculty of Mechanical Engineering
advertisement

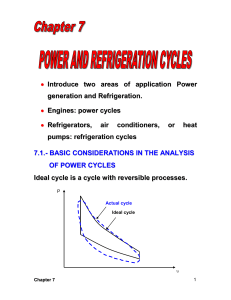
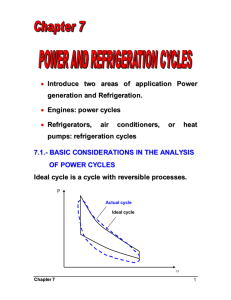
1 Chapter 1 Gas Power Cycles – Reciprocating Engines A power cycle refers to power generation which is accomplished by a system that operates on a thermodynamic cycle. The system or device is often called an engine. In gas cycles, the working fluid remains gaseous throughout the entire cycle. Examples of gas power cycles are : internal combustion engine (ICE) and gas-turbine engine. 1.1 The Carnot cycle The Carnot cycle is the most efficient power cycle. It is an ideal cycle with thermal efficiency expressed as T th,Carnot 1 L TH when operating between a heat source at temperature TH and a sink at temperature TL. Thermal efficiency increases with an increase in TH or with a decrease in TL, which are also true for actual cycle. In practice, the highest temperature in the cycle is limited by the metallurgical limits of the components (piston, cylinder wall, turbine blade, etc.). In an actual cycle, the lowest temperature is limited by the temperature of the cooling medium used in the cycle such as a lake, a river, or the atmospheric air. 1.2 Air-standard assumptions In internal combustion engines energy (heat) is obtained by burning fuel within the system boundaries the combustion process changes the composition of the working fluid from air and fuel to combustion products All internal combustion engines operate on a mechanical cycle; the working fluid does not undergo a complete thermodynamic cycle work on an open cycle The actual gas power cycles are complex. To simplify the analysis it is common to utilize the air-standard assumptions: the working fluid is air, which continuously circulates in a closed loop and always behaves as an ideal gas all the processes that make up the cycle are internally reversible the combustion process is replaced by a heat-addition process from an external source the exhaust process is replaced by a heat-rejection process that restores the working fluid to its initial state When assuming the air has constant specific heats determined at room temperature of 25 oC, the air-standard assumptions become cold-air-standard assumptions. A cycle using the air-standard assumptions is known as an air-standard cycle. 2 1.3 Overview of reciprocating engines A reciprocating engine is a piston-cylinder device which is simple with a wide range of applications. It is used in automobiles, light aircraft, ships and many other devices. Intake valve Exhaust valve Bore TDC piston stroke connecting rod BDC crankshaft In a reciprocating engine, the piston reciprocates in the cylinder between top dead center (TDC) and bottom dead center (BDC). The distance between TDC and BDC is the stroke of the engine. The diameter of the piston is known as the bore. The air or air-fuel mixture is drawn into the cylinder through the intake valve, and the combustion gases are expelled from the cylinder through the exhaust valve. When the piston is at TDC, the minimum volume formed in the cylinder is called the clearance volume. The volume displaced by the piston as it moves between TDC and BDC is known as the displacement volume. The ratio of the maximum volume formed in the cylinder to the minimum volume is the compression ratio of the engine: r Vmax VBDC Vmin VTDC (Note : this is not to be confused with the pressure ratio) The mean effective pressure (MEP) is a fictitious pressure, that if it acted on the piston during the entire power stroke, would produce the same amount of net work as that produced during the actual cycle. Wnet = MEP x Piston area x Stroke = MEP x Displacement volume or MEP = Wnet wnet Vmax Vmin vmax vmin ………. (kPa) The engine with a larger value of MEP delivers more net work per cycle and therefore is a better performing engine. 3 P Wnet MEP V Vmin TDC Vmax BDC Fig. 1.1 Graphical representation of the mean effective pressure (MEP) Reciprocating engines can be classified as: Spark-ignition (SI) engines – combustion is initiated by a spark plug Compression-ignition (CI) engines – air-fuel mixture is self-ignited as a result of compressing the mixture above its self-ignition temperature. The Otto cycle is an ideal cycle for SI engines, whereas the Diesel cycle is an ideal cycle for CI engines. 1.3.1 Four-stroke engines Figure 1.2 shows the actual and ideal cycles in four-stroke SI engines and their P-v diagrams. 1 – 2 Induction stroke: The piston travels from TDC to BDC and air-fuel mixture is drawn into the cylinder through the intake valve. Note that pressure in the cylinder is slightly below atmospheric. 2 – 3 Compression stroke: The piston moves from BDC to TDC, compressing the airfuel mixture. Just before reaching TDC, the spark plug fires and the mixture ignites, increasing the pressure and temperature of the system. 3 – 4 Expansion stroke: The high-pressure gases force the piston down from TDC to BDC, forcing the crankshaft to rotate, producing useful work output during this stroke which is also known as power stroke. 4 – 1 Exhaust stroke: The piston moves from DBC to TDC, purging the exhaust gases through the exhaust valve. During this stroke, the pressure in the cylinder is slightly above atmospheric. It takes 4 strokes (and 2 crankshaft revolutions) to complete 1 “thermodynamic” cycle (actually mechanical cycle). 4 1.3.2 Two-stroke engines In two-stroke engines, the induction and exhaust strokes are part of the compression stroke, as shown by the timing diagram. On a timing diagram, the angular position is in terms of crank angle position in relation to TDC and BDC positions. For clockwise rotation, moving from BDC to TDC (compression stroke), induction, exhaust and transfer (air-fuel mixture from crankcase to cylinder) processes are part of the compression stroke. Similarly, during the expansion stroke (moving from TDC to BDC), induction, exhaust and transfer (air-fuel mixture from crankcase to cylinder) processes are also part of the expansion stroke. Two-stroke engines are less efficient than four-stroke engines due to incomplete expulsion of exhaust gases and partial expulsion of fresh air-fuel mixture with exhaust gases Two-stroke engines are relatively simple and inexpensive Two-stroke engines have high power-to-weight and power-to-volume ratios, which make them suitable for applications that require small size and weight (e.g. motorcycles, chain saws, lawn mowers etc.) 1.4 Otto cycle – ideal cycle for SI engines The Otto cycle is the ideal cycle for SI reciprocating engines. Most SI engines are four-stroke internal combustion engines. Figure 1.3 shows the Otto cycle on a P-v and T-s diagrams. This cycle closely resembles the actual four-stroke or two-stroke cycles of SI engines. P T 3 3 . qin isentropic expansion qin v=C 2 4 2 4 qout isentropic compression qout 1 v=C 1 v TDC BDC Figure 1.3 P-v and T-s diagrams for Otto cycle The (air-standard) Otto cycle consists of 4 internally reversible processes: 1–2 2–3 3–4 4–1 Isentropic compression Constant-volume heat addition Isentropic expansion Constant-volume heat rejection s 5 The cycle is executed in a closed system, and ignoring changes in kinetic and potential energies, the energy balance for any of the processes, on a unit-mass basis is qin qout ( win wout ) u ……… (kJ/kg) Processes 2 – 3 and 4 – 1 take place at constant volume, thus the work terms are zero. Therefore, qin u3 u 2 cv T3 T2 and qout u 4 u1 cv T4 T1 Using cold air standard assumptions, the thermal efficiency of the ideal Otto cycle is th,Otto wnet qin qout q T T 1 out 1 4 1 qin qin qin T3 T2 Processes 1 – 2 and 3 – 4 are isentropic, and v2 = v3 and v4 = v1. Therefore, T1 v 2 T2 v1 k 1 v 3 v4 k 1 T4 T3 i.e., T2 T1 r k 1 where r is the compression ratio ( r th,Otto 1 and T3 T4 r k 1 cp vmax v1 ) and k . Thus, cv vmin v2 T4 T1 T4 T1 1 1 1 k 1 k 1 k 1 T3 T2 T4 r T1 r r The thermal efficiency increases with both r and k. This is also true for actual SI internal combustion engines. For a given r, the thermal efficiency of an actual cycle is less than that of the ideal Otto cycle due to irreversibilities (friction) and incomplete combustion in real engines. Typical values of r for real SI engines range from 7 through 10. Example 1a (constant sepcific heats) The compression ratio of an ideal Otto cycle is 8. At the start of compression, air is at 100 kPa, 17 C, and 800 kJ/kg of heat is transferred to the air during the constantvolume heat addition process. Assuming cv = 0.718 k and k = 1.4, determine (a) the maximum temperature and pressure in the cycle, (b) the net work output, (c) the thermal efficiency, and (d) the mean effective pressure for the cycle. k 1 v (a) T2 T1 1 (17 273)8 0.4 = 666.3 K v2 qin u3 u 2 cv T3 T2 800 = 0.718 (T3 666.3) T3 = 1780.5 K 6 k v P2 P1 1 100(8)1.4 = 1837.9 kPa v2 T 1780.5 = 4911.3 kPa P3 P2 3 1837.9 666.3 T2 k Pmaz v 1 P4 P3 3 4911.3 = 267.2 kPa 8 v4 P 267.2 T4 T1 4 290 = 774.9 K P 100 1 1.4 (b) qin = 800 kJ/kg (given) qout u 4 u1 cv T4 T1 = 0.718(774.9 – 290) = 348.2 kJ/kg wnet = qin – qout = 800 – 348.2 = 451.8 kJ/kg w 451.8 (c) Otto net = 0.565 qin 800 RT 0.287(290) (d) v1 1 = 0.8323 m3/kg P1 100 v 0.8323 = 0.1040 m3/kg v2 1 r 8 wnet 451.8 = 620.3 kPa MEP v1 v2 0.8323 0.1040 Example 1b (variable specific heats) The compression ratio of an ideal Otto cycle is 8. At the start of compression, air is at 100 kPa, 17 C, and 800 kJ/kg of heat is transferred to the air during the constantvolume heat addition process. Accounting for the variation of specific heats of air with temperature, determine (a) the maximum temperature and pressure in the cycle, (b) the net work output, (c) the thermal efficiency, and (d) the mean effective pressure for the cycle. Assume air standard assumptions Kinetic and potential energy changes are negligible Specific heats vary with temperature (use table for air). P 3 . isentropic expansion qin qin = 800 kJ/kg r =8 2 4 qout isentropic compression 1 v TDC BDC P1 = 100 kPa T1 = 290 K 7 T1 = 290 K (using Table A-17) Process 1 – 2 is isentropic, u1 = 206.91 kJ/kg, vr1 = 676.1 v2 vr 2 1 v1 v r1 r v r1 676 .1 = 84.51 (Table A-17) T2 = 652.4 K, u2 = 475.11 kJ/kg r 8 T v p v pv 652.4 P2 P1 2 1 = 100 R 2 2 1 1 8 = 1799.7 kPa T2 T1 290 T1 v 2 vr 2 or Process 2 – 3 is constant-volume heat addition: qin = u3 – u2 800 = u3 – 475.11 u3 = 1275.11 (Table A-17) T3 = 1575.1 K R T P3 P2 3 T2 p 3 v3 p 2 v 2 T3 T2 , vr3 = 6.108 v 2 1575 .1 = 1799 .7 1 = 4345 kPa 652 .4 v3 (b) Process 3 – 4 is isentropic expansion, v4 vr 4 r v3 v r 3 v r 4 rv 3 8 (6.108) = 48.864 Table A-17 gives T4 = 795.6 K, u4 = 588.74 kJ/kg qout = u4 – u1 = 588.74 – 206.91 = 381.83 kJ/kg wnet,out = qnet,in = qin – qout = 800 – 381.83 = 418.17 kJ/kg (c) th wnet ,out (d) v1 RT1 0.287(290) = 0.832 m3/kg P1 100 qin MEP 418 .17 = 0.523 @ 52.3 % 800 wnet wnet wnet 418 .17 = 574 kPa v v1 v 2 1 1 1 v1 v1 1 0.8321 r r 8 i.e A constant pressure of 574 kPa during the power stroke would produce the same amount of work output as the entire cycle. 1.5 Diesel Cycle – ideal cycle for compression-ignition engines In SI (gasoline) engines, air-fuel mixture is compressed to a temperature below the autoignition temperature of the fuel, and the combustion process is initiated by firing a spark plug. In CI (i.e. diesel) engines, the air is compressed to a temperature above the autoignition temperature of the fuel, and combustion takes place as the fuel is injected into this hot air. The spark plug and carburetor (in SI engines) is replaced with a fuel injector in diesel engines. 8 The compression ratios in SI engines are limited by the onset of autoignition or engine knock. However, diesel engines can operate at higher compression ratios (typically between 12 and 24), because only air is compressed during the compression stroke. Fuel is injected in diesel engines when the piston approaches TDC and continues during the first part of the power stroke. That is, the combustion takes place over a longer interval. Thus, the combustion process in ideal Diesel cycle is modelled as a constant-pressure heat-addition process. (note that in Otto cycle heat addition is at constant volume). The Otto and Diesel cycles differ only on the heat-addition process. The other processes are the same for both ideal cycles. P qin 2 T P=C 3 . 3 qin 2 isentropic expansion V=C 4 qout isentropic compression 4 qout 1 1 TDC v BDC P-v and T-s diagrams for ideal Diesel cycle s The Diesel cycle is executed in a piston-cylinder device, which forms a closed system. Th cycle consists of 4 internally reversible processes: 1–2 2–3 3–4 4–1 Isentropic compression Constant-pressure heat addition Isentropic expansion Constant-volume heat rejection The energy balance for process 2 – 3 is written as qin wb ,out u 3 u 2 qin P2 v3 v2 u3 u 2 = h3 h2 c p T3 T2 The heat rejection (4 – 1) is similar to the ideal Otto cycle qout u 4 u1 cv T4 T1 The thermal efficiency of the ideal Diesel cycle under the cold-air-standard assumptions is 9 th, Diesel wnet qin qout q c T T 1 out 1 v 4 1 qin qin qin c p T3 T2 = 1 T4 T1 k T3 T2 The cutoff ratio rc is defined as rc T2 T1 r k 1 P3 v3 RT3 P2 v 2 RT 2 process 1 – 2 : process 2 – 3 : V3 v3 V2 v 2 T3 v3 rc T2 v2 gives T3 rc T2 rc T1 r k 1 process 3 – 4 : Tv k 1 v T C …… 4 3 T3 v 4 r T4 T3 c r Therefore, th, Diesel = 1 When rc 1, k 1 rcT1r T4 T1 k T3 T2 th, Diesel k 1 k 1 rc r v 3 v1 k 1 v3 v 2 v 1 v 2 k 1 r c r k 1 k 1 rck T1 1 r k 1 1 rck 1 = 1 k c1 1 k rc r r k 1 r k 1 k rc 1 1 1 k 1 (see Richard Stone, “Introduction to r internal combustion engines”) Example 2 An ideal Diesel cycle has a compression ratio of 18 and a cutoff ratio of 2. At the start of the compression process, the working fluid is at 0.1 Mpa and 27 oC. Utilizing the cold-air-standard assumptions, determine (a) the thermal efficiency, and (b) the mean effective pressure. Data: P1 = 100 kPa, T1 = 300 K, r v v1 18 , rc 3 2 v2 v2 k 1 v (a) T2 T1 1 300(18) 0.4 = 953.3 K v2 v T3 T2 3 953.3(2) 1906.6 K v2 v T4 T3 3 v4 k 1 v3 v 2 T3 v 4 v2 k 1 2 1906.6 18 0.4 = 791.4 K 10 qin = cp (T3 – T2) = 1.005 (1906.6 – 953.3) = 958.1 kJ/kg qout = cv (T4 – T1) = 0.718 (791.4 – 300) = 352.5 kJ/kg qin qout 958.1 352.8 = 0.632 @ 63.2 % qin 958.1 RT 0.287(300) (b) v1 1 = 0.8610 m3/kg P1 100 v 0.8610 = 0.0478 m3/kg v2 1 r 18 wnet 958.1 352.8 = 744.3 kPa MEP v1 v2 0.8610 0.0478 th, Diesel =