WS 14.1 groupwork - sarahhockenchemistry
advertisement

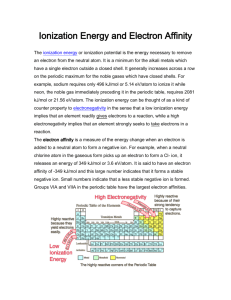
WS 14.1 Introduction to Periodic Trends (Group activity) period# _____ Group # ______ 1. In general, opposite charges will be attracted to each other (there will be an “attractive force” between the two particles), and if two particles have the same charge, there will be a “repulsive force” between the two particles. Keep this in mind as you answer the questions on this worksheet. a. Is the force between an atom’s electrons and its nucleus attractive, or repulsive? (which one?) ____________ Explain why. b. Is the force between electrons and other electrons in the atom attractive, or repulsive? ______________ Explain why. c. As two charged objects get closer together, does the attractive or repulsive force increase or decrease? ___________ d. The higher the charge on two charged objects, the stronger / weaker the attractive or repulsive force between the objects will be. (Circle the answer) e. As n-level of an electron increases, does the electron get closer to or further from the nucleus?________ WS 14.1 Introduction to Periodic Trends (Group activity) period# _____ Group # ______ 1. In general, opposite charges will be attracted to each other (there will be an “attractive force” between the two particles), and if two particles have the same charge, there will be a “repulsive force” between the two particles. Keep this in mind as you answer the questions on this worksheet. a. Is the force between an atom’s electrons and its nucleus attractive, or repulsive? (which one?) ____________ Explain why. b. Is the force between electrons and other electrons in the atom attractive, or repulsive? ______________ Explain why. c. As two charged objects get closer together, does the attractive or repulsive force increase or decrease? ___________ d. The higher the charge on two charged objects, the stronger / weaker the attractive or repulsive force between the objects will be. (Circle the answer) e. As n-level of an electron increases, does the electron get closer to or further from the nucleus?________ 2. Trends for Atomic Radius. (Refer to the pictures and numbers as you answer the questions.) p.____ group #____ (The atomic radii of Li, Na, K, F, Cl, and Br are reported in nanometers.) (Pictures of row 2 and row 3) and Ca and K. Write the nm value next to Li, Na, K, F, Cl Li Na K .155 nm 0.190 nm .235 nm F 0.057 nm Cl 0.097 nm Br 0.112 nm a. As you go down a column on the periodic table, what happens to the radius of the elements?__________________ b. Write the electron configuration of each atom (OK to use the noble gas abbrevation) Li Na K c. Explain why the radius increases or decreases as you go down a column. Refer to the elements/configurations to (b) as part of your answer. d. As you go from left to right across a row on the periodic table, what happens to the radius of the elements?______________________ e. Write the electron configuration for fluorine. f. Why does the radius increase/decrease as you go from the left to right across a row? Refer to lithium and fluorine as part of your explanation. The concepts in #1 are very relevent here! 3. For each pair of atoms, circle the atom with the larger radius. Mg or Ca Ca or S Zn or Br Ne or He Rb or Ca 4. Atom or ion K K+1 Cl Cl-1 Mg Mg+2 O O-2 Radius (nm) 0.235 0.152 0.097 0.167 0.160 0.086 0.073 0.126 period ____ group # _____ Assumining that other ions follow the trend in the above chart (they do!), circle the correct words: a. When an atom gains / loses electron(s) to form a cation, the cation that forms has a radius that is larger / smaller than the original atom’s radius. b. When an atom gains / loses electron(s) to form an anion, the anion that forms has a radius that is larger / smaller than the original atom’s radius. 5. For each pair, circle the larger atom or ion. a. Cu or Cu+2 b. N or N-3 c. Pb+4 or Pb Cl-1 or Cl d. e. calcium atom or calcium ion f. sulfur atom or sulfide ion 6. For each set of atoms or ions, circle the atom or ion with the larger radius, and put a box around the atom or ion with the smallest radius. a. As , As+5 , As-3 b. Be C c. S-2 Cl d. Cl Sr Mg e. Se Zn Ba F f. Ti Ti+2 K O S P Part II. Ionization Energy! Ionization Energy (IE) = The energy required to remove an electron from a gas phase atom or ion. (First Ionization energy (IE1) = the energy required to remove the first electron from an atom, to form a +1 ion) (Second Ionization Energy (IE2) = the energy required to remove the second electron, to go from a +1 ion to a +2 ion.....) (FYI there is another term, called Electron Affinity, which measures the energy change when an atom GAINS an e-, to form an anion) Element Na K Rb Al Ca O Cl S F 1st ionization energy (kJ/mole) 496 418 403 577 589 1310 1250 999 1684 Chlorine has an ionization energy (IE1) of 1250 kJ/mole. The following equation represents the first ionization of chlorine: Cl(g) + 1250 kJ -------> Cl+1(g) + 1 e- 7 a. What is the ionization energy of sodium?_____ b. Write an equation representing the first ionization of sodium, and include the heat term. Include subscripts. (use the chlorine reaction, above, as an example if needed.) c. What is the first ionization energy of sulfur?_______ d. Write an equation representing the first ionization of sulfur, and include the heat term. Include subscripts. e. Out of Na, Cl, and S, which one of these elements is most likely to be found as a positive ion, in nature?________ f. Do the elements that typically form positive ions tend to have a relatively high or low ionization energy?_____ g. Are the elements in (f) more likely to be metals or nonmetals?_______ 8. How ionization energy is related to the periodic table: Based on the data given in problem 7 (assuming that other elements follow the same trends): a. As you go down a column on the periodic table, what happens to the ionization energy of elements?_____________ b. Pick examples from 2 different columns on the periodic table that support your answer. Include numbers! 1st example: 2nd example: c. Fill out: Element Li Na K Electron configuration [He]2s1 ________________ ________________ 1st ionization energy: 520 kJ . _____________ _____________ d. Explain why ionization energy increases or decreases as you go down a column on the periodic table. Be sure to refer to the concepts from #1 as you answer this! e. Fill out: Element Na S Cl # of protons ________ ________ ________ radius(nm) 0.190 0.127 0.097 1st ionization energy ___________ ___________ ___________ f. As you go from left to right across a row on the periodic table, what happens to the ionization energy of elements? g. Explain why ionization energy increases or decreases as you go from left to right across a row. (Be sure to refer to concepts from #1!) 9. Rewrite each list of elements in order, from smallest radius to largest radius, and from lowest ionization energy to highest ionization energy. low R to high R low IE to high IE Li C ______________ ______________ N, P ______________ ______________ Mg S Al ______________ ______________ Ti Ti+4 Ti+2 ______________ ______________ Br-1, F, Br ______________ ______________ ______________ ______________ ______________ ______________ ______________ ______________ Sr+2 Cs F Cl Mg Sr Te Se O 10. Isoelectronic Series: a. Fill in the blanks Ion formula: N-3 O-2 Radius (nm) 0.171 0.126 Number of protons: ______ ______ Number of electrons: ______ ______ F-1 Na+1 Mg+2 Al+3 0.116 0.096 0.086 0.050 _____ ______ _____ ______ _____ ______ _____ ______ b. This series of ions is “isoelectronic.” What does it mean to be isoelectronic? (hint: “iso” means “same”) c. Which noble gas is “isoelectronic” with these ions? ________ d. Explain why the radius decreases as you go from left to right in the above series of ions. e. For each pair, circle the ion that you would expect to have a larger radius, and indicate which noble gas they are isoelectronic with. Cl-1 or Ca+2 As-3 or Se-2 Ca+2 or K+1