practice test ch 7 & 8 Multiple Choice Identify the choice that best
advertisement

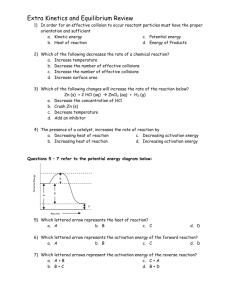
practice test ch 7 & 8 Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. Which of the following best describes the rates of chemical reaction? a. most chemical reactions occur very slowly b. most chemical reactions occur at moderate rates c. most chemical reactions occur very rapidly d. chemical reactions have a wide range of rates, from extremely fast to extremely slow ____ 2. Which of the following is true of the rates of most chemical reactions? a. the initial rate is cannot be measured b. the initial rate is faster than the rate later in time c. the initial rate is the same as the rate later in time d. the initial rate is slower than the rate later in time ____ 3. Which of the following is true of effective collisions? a. the number of effective collisions decreases as the temperature is increased b. the number of effective collisions determines the reaction rate c. both a. and b. d. Neither a. nor b. ____ 4. Which of the following is true of effective collisions? a. the number of effective collisions determines the reaction rate b. the number of effective collisions increases as the temperature is increased c. both a. and b. d. Neither a. nor b. ____ 5. Which of the following is true? a. decreasing the activation increases the reaction rate b. the activation energy for an exothermic reaction is negative c. both a. and b. d. Neither a. nor b. ____ 6. In an energy diagram for a chemical reaction what species appears at the highest energy? a. the catalyst c. the reactants b. the products d. the transition state ____ 7. Given that the reaction 2 H2(g) + O2(g) 2 H2O(g) is exothermic, which of the following is true of the reaction 2 H2O(g) 2 H2(g) + O2(g) ? a. its activation energy is lower than that of 2 H2(g) + O2(g ) ? 2 H2O(g) b. its activation energy is the same as that of 2 H2(g) + O2(g ) ? 2 H2O(g) c. its activation energy is higher than that of c. its activation energy is the same as that of 2 H2(g) + O2(g ) ? 2 H2O(g) d. there is no relationship between its activation energy and that of 2 H2(g) + O2(g ) ? 2 H2O(g) ____ 8. In an energy diagram for an endothermic chemical reaction which of the following is true? a. the energy of the products is lower than that of the reactants b. the energy of the transition state is higher than that of the reactants c. both a. and b. d. Neither a. nor b. ____ 9. Consider a reaction such as A2(g) + B2 (g) 2AB(g). What can we say about the mechanism of the reaction? a. if the reaction is fast it occurs in a single step b. if the reaction is fast it occurs in multiple steps c. if the reaction is slow it occurs in multiple steps d. we must examine each case individually ____ 10. Which of the following accounts for the fact that reactions between ions in solution are usually very fast? a. ionic bonds are weak b. no covalent bonds need to be broken for reaction to occur c. reactions between ions are endothermic d. reactions between ions are exothermic ____ 11. Sodium chloride and silver nitrate react to produce silver chloride and sodium nitrate? Which of the following will result in the fastest reaction between sodium chloride and silver nitrate? a. crystals of the two reactants are placed in a mortar and ground up b. large crystals of the two reactants are placed in contact with one another c. powdered samples of the two reactants are mixed together d. solutions of the two reactants are mixed together ____ 12. If a chemist wishes to carry out a reaction in which one reactant is a solid and one is a liquid, which of the following will be most effective in speeding up the reaction? a. adding more liquid reactant b. grinding the solid to a form a powder c. placing a larger crystal of the solid in the same volume of liquid reactant d. all of the above will be equally effective ____ 13. When the concentration of a reactant is increased which of the following is true? a. the rate of reaction always decreases c. the rate of reaction always increases b. the rate of reaction usually decreases d. the rate of reaction usually increases ____ 14. If a particular reactant is involved in the slowest step of a multi-step reaction what will be the effect of increasing its concentration? a. the reaction rate will always decrease b. the reaction rate will usually decrease c. the reaction rate will always increase d. the reaction rate will usually increase ____ 15. A particular chemical reaction carried out at 20°C takes 2 hours. Approximately how long will it take to carry out the reaction at 40°C? a. 0.5 hour c. 4 hours b. 1 hour d. 8 hours ____ 16. A catalyst speeds up a chemical reaction as a result of which of the following? a. it increases the number of collisions between reacting molecules b. it makes the reaction more endothermic c. it makes the reaction more exothermic d. it provides an alternative reaction pathway ____ 17. Which of the following is true of fevers? a. an increase in body temperature never has beneficial effects b. a 1°C increase in temperature can be protective c. a 3°C increase in temperature is invariably fatal d. none of the above is true ____ 18. Which of the following statements is true about a reversible reaction? a. All reversible reactions are exothermic. b. All reversible reactions have low activation energy. c. When we mix the reactants together one or more of the reactants will be completely used up. d. When we mix the reactants together none of the reactants will be completely used up. ____ 19. Which of the following an example of an equilibrium situation? a. an unsaturated solution c. a supersaturated solution b. a saturated solution d. all of the above ____ 20. Consider a sample of water in a closed container. When the reaction equilibrium what can we say about any specific water molecule? a. if it was present in the liquid it will always remain in the liquid b. if it was present in the vapor it will always remain in the vapor c. it will sometimes be in the liquid phase and sometimes in the vapor phase d. both a. and b. are true has reached ____ 21. In writing the equilibrium constant expression we use square brackets. The notation [A] means which of the following? a. the mass of A c. the molar concentration of A b. the number of moles of A d. none of the above ____ 22. Which of the following must we know in order to write the equilibrium constant for a chemical reaction? a. the balanced chemical equation for the reaction b. the rate at which the reaction occurs c. both a. and b. d. Neither a. nor b. ____ 23. For the reaction expression? a. K = [H2][N2]/[NH3] b. K = [NH3]/[H2][N2] which of the following is the equilibrium constant c. K = [H2]3[N2]/[NH3]2 d. K = [NH3]2/[H2]3[N2] ____ 24. For the reaction at 25C equilibrium is established when [NOCl] = 2.6 M, [NO] = 1.4 M and [Cl2] = 0.34 M. What is the equilibrium constant for this reaction? a. 0.099 c. 5.4 b. 0.18 d. 10 ____ 25. Which of the following could be the equilibrium constant for a reaction which does not yield a significant concentration of products? a. 1 x 10-15 c. 1 x 10 2 b. 1 x 10-2 d. 1 x 10 15 ____ 26. Which of the following will change the numerical value of the equilibrium constant of a particular reaction? a. increasing the concentration of reactants b. increasing the concentration of products c. increasing the temperature d. all of the above ____ 27. A particular reaction has an equilibrium constant of 1 x 10 20. Which of the following best describes the relationship between the equilibrium constant and the reaction rate? a. because the equilibrium constant is large the reaction proceeds rapidly b. there is no relationship between the size of the equilibrium constant and the rate of the reaction c. both a. and b. are true d. Neither a. nor b. is true ____ 28. Le Chatelier’s Principle applies to which of the following systems? a. a system in which the reaction is moving left to right b. a system in which the reaction is moving right to left c. a system which has reached equilibrium d. all of the above ____ 29. If the endothermic reaction the temperature of the reaction vessel? a. the reaction will shift left to right has reached equilibrium, what is the effect of lowering b. the reaction will shift right to left c. there will be no further reaction d. what happens depends on the temperature ____ 30. For the exothermic reaction which of the following changes could be carried out in such a way as to not cause the reaction to shift either to the left or to the right? a. adding H2 and removing NH3 b. adding H2 and lowering the temperature c. adding H2 and raising the temperature d. adding H2 and decreasing the volume ____ 31. When considering the effect of a catalyst on a system to which Le Chatelier’s Principle can be applied, which of the following is true? a. addition of a catalyst has absolutely no effect on the reaction b. addition of a catalyst speeds up the forward reaction c. addition of a catalyst speeds up the reverse reaction d. addition of a catalyst speeds up both the forward and reverse reaction by the same amount ____ 32. The species H3O+ can be called which of the following? a. heavy water c. hydronium ion b. hydrogen ion d. all of these ____ 33. Which of the following occurs when NaOH is dissolved in water to form a basic solution? a. a complex between NaOH and water is formed b. NaOH breaks up to form Na+(aq), H+(aq) and O2-(aq) c. NaOH breaks up to form Na+(aq) and OH-(aq) d. the water molecule loses an H+ ____ 34. Which of the following can be characterized as a strong acid? a. HNO3 c. both a. and b. b. H2SO4 d. Neither a. nor b. ____ 35. Which of the following can be characterized as a strong base? a. LiOH c. both a. and b. b. KOH d. Neither a. nor b. ____ 36. Which of the following acids is the acid found in gastric fluid? a. acetic c. nitric b. hydrochloric d. sulfuric ____ 37. Which of the following can be characterized as a strong electrolyte? a. 0.001 M HCl c. both a. and b. b. 3.0 M HCl d. Neither a. nor b. ____ 38. Which of the following can be characterized as a strong electrolyte? a. 0.001 M KOH c. both a. and b. b. 3.0 M NH3 d. neither a. nor (b ____ 39. Which of the following is the Brønsted-Lowry definition of a base? a. a hydroxide ion acceptor c. a proton donor b. a proton acceptor d. none of these ____ 40. Which of the following is the conjugate base of water? a. H3O+ c. OHb. NaOH d. O2____ 41. Which of the following is not an acid? a. H3O+ b. H2CO3 c. HPO42d. none, they are all acids ____ 42. Which of the following is a triprotic acid? a. HCl b. HNO3 c. H2SO4 d. H3PO4 ____ 43. Which of the following is the conjugate acid of PO43-? a. H3PO4 c. HPO42b. H2PO4d. H3O+ ____ 44. Which of the following is the conjugate base of H2PO4-? a. H3PO4 c. PO43b. HPO42d. OH____ 45. Which of the following statements best describes the acid/base relationship? a. as acid strengths decrease conjugate base strengths increase b. as base strengths decrease conjugate acid strengths decrease c. both a. and b. d. neither a. and b. ____ 46. What is the name of the acid which dissociates to give the cyanide ion, CN-? a. cyanic acid c. cyanous b. cyanidic acid d. hydrocyanic acid ____ 47. What is the name of the acid which dissociates to give the nitrite ion, NO2-? a. hydronitric acid c. nitritic acid b. nitric acid d. nitrous acid ____ 48. In an acid-base equilibrium the equilibrium lies in the direction which favors which species? a. the stronger acid c. both a. and b. b. the stronger base d. Neither a. nor b. ____ 49. In an acid-base equilibrium the equilibrium lies in the direction which favors which species? a. the weaker acid c. both a. and b. b. the weaker base d. Neither a. nor b. ____ 50. When HCl is dissolved in water which of the following is true of the equilibrium which is established? a. it lies virtually completely to the left c. it lies virtually completely to the right b. it lies slightly to the left d. it lies slightly to the right ____ 51. When comparing two acids which of the following is true? a. the stronger acid has the larger Ka c. both a. and b. b. the stronger acid has the larger pKa d. Neither a. nor b. ____ 52. The Ka for boric acid is 7.3 x 10-10. What is the pKa of boric acid? a. -9.14 c. 4.86 b. -4.86 d. 9.14 ____ 53. When an acid reacts with a base the reaction is called which of the following? a. neutralization c. redox b. precipitation d. single displacement ____ 54. Which of the following is the [H+] in pure water at room temperature? a. 1.0 x 10-14 M c. 1.0 x 107 M -7 b. 1.0 x 10 M d. 1.0 x 1014 M ____ 55. In an aqueous solution thee [OH-] is 1.0 x 10- 6 M. What is the [H+]? a. 1.0 x10 -9 M c. 1.0 x 10 - 6 M -8 b. 1.0 x 10 M d. 1.0 x 10 8 M ____ 56. Which of the following is the definition of pH? a. 1 x 10pH c. - log[H3O+] + b. log[H3O ] d. none of these ____ 57. Which of the following is the correct relationship between pH and pOH? a. (pH) x (pOH) = 14 c. pH + pOH = 14 b. pH/pOH = 14 d. pH pOH = 14 ____ 58. A solution has a pH of 5.4. What is its pOH? a. -8.6 b. -5.4 c. 5.4 d. 8.6 ____ 59. The equivalence point of a titration corresponds to which of the following? a. the point where equal volumes of acid and base have been used b. the point where the acid and base have been added in proper stoichiometric amounts c. the point where a pH indicator changes color d. none of the above ____ 60. The end point of an acid-base titration corresponds to which of the following? a. the point where equal volumes of acid and base have been used b. the point where the acid and base have been added in proper stoichiometric amounts c. the point where a pH indicator changes color d. none of the above ____ 61. Which of the following solutions will require the most NaOH in a titration experiment? a. 25 mL of 0.5 M HCl c. 25 mL of 0.5 M HC2H3O2 b. 25 mL of 0.5 M HCOOH d. they all require the same amount ____ 62. In a particular titration experiment a 30.0 mL sample of an unknown monoprotic acid required 25.0 mL of 0.200 M NaOH for the end point to be reached. What is the concentration of the acid? a. 0.120 M c. 0.240 M b. 0.167 M d. 0.333 M ____ 63. Which of the following is characteristic of a buffer? a. the pH will go down significantly when H3O+ is added to the buffer b. the pH will go down very slightly when H3O+ is added to a buffer c. the pH will go up significantly when H3O+ is added to the buffer d. the pH will go up very slightly when H3O+ is added to the buffer ____ 64. Which of the following solutions can function as a buffer? a. a solution containing HCl and NaCl b. a solution containing NaOH and NaCl c. a solution containing HC2H3O2 and NaC2H3O2 d. all of the above ____ 65. Human blood contains which of the following buffers? a. H2CO3/HCO3c. both a. and b. b. H2PO4-/HPO42d. Neither a. nor b. practice test ch 7 & 8 Answer Section MULTIPLE CHOICE 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. D B B C A D C B D B D B D D A D B D B C C A C A A C B C A C D C C C B B C A B C D 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. 63. 64. 65. D C B A C D D C C A D A B B C C D B C D B B C C