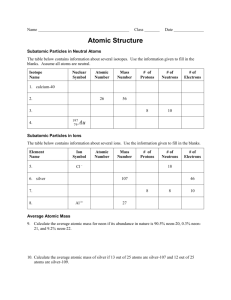
Atomic Mass = (% isotope #1)(mass of #1) +(% isotope #2)(mass of
advertisement

Notes Wkst – History of Atom Atomic Mass = (% isotope #1)(mass of #1) +(% isotope #2)(mass of #2) + (% isotope #3)(mass of #3) + . . . . . (Please notice that even though you are finding an average, you do NOT divide by the number of isotopes. The percentages of abundance take care of this for you. You must change the percentages to decimals before putting them into the equation.) Example 1 Naturally occurring chlorine is 75.53% chlorine-35 and 24.47% chlorine-37. What is the average atomic mass which should be placed on the Periodic Table for the element chlorine? (.7553 x 35) + (.2447 x 37) = avg. atomic mass Example 2 The element neon consists of three isotopes with masses 19.99, 20.99 and 21.99 amu’s. These three isotopes are present in nature to the extent of 90.92%, 0.25% and 8.83% respectively. Calculate the atomic mass of neon Chemical Configuration is a way of displaying atoms or ions showing lots of information Example 3 Write the chemical configuration for a particle containing 5 protons, 6 neutrons and 5 electrons. Example 4 Write the chemical configuration for a particle which contains 8 neutrons, has a mass number of 17 and contains 10 electrons. Example 5 Write the chemical configuration for a particle which contains 18 electrons, has a mass number of 32 and has a –2 charge. Example 6 Write the chemical configuration for a particle which has a charge of +3 and contains 10 electrons and 14 neutrons. Example 7 Write the chemical configuration for a particle which has a charge of +2, an atomic number of 24 an contains 28 neutrons Example 8 Write the chemical configuration for a particle which has a mass number of 31, contains 18 electrons and has a –3 charge. Sometimes you are not given enough information to write the entire chemical configuration for a particle, but you can at least identify it as to what element it is and whether it is an atom or an ion. Example 9 What particle has a +2 charge and 12 protons? What particle has 50 protons and 48 electrons? What particle has 44 protons and 44 electrons? What particle has a –3 charge and contains 36 electrons?