CHEMISTRY I CHAPTER 4, #2 1. Compare and contrast atomic
advertisement

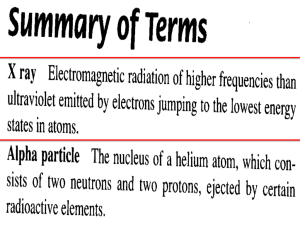
NAME: __________________________ CHEMISTRY I CHAPTER 4, #2 1. Compare and contrast atomic number and mass number of an element. 2. Can atoms of 2 different elements have the same atomic number? EXPLAIN WHY OR WHY NOT. 3. Can atoms of 2 different elements have the same mass number? EXPLAIN WHY OR WHY NOT. 4. The common isotope of carbon has a mass number of 12. However, isotopes of carbon are known that have mass numbers of 13 and 14. Why are they all considered carbon atoms, even though they differ in mass? (Questions 5-9) Write the atomic symbol (AZX) for each of the following: 5. Z = 6, number of neutrons = 7 __________________ 6. Z = 6, A = 13 __________________ 7. Z = 19, number of neutrons = 25 __________________ 8. the isotope of calcium with a mass number of 41 9. the isotope with 19 protons and 16 neutrons __________________ Complete the following table: SYMBOL PROTONS 41 20Ca 15 134 Cs 56 35 73 __________________ NEUTRONS MASS NUMBER 16 82 179 10. Sodium-24 decays by the loss of a beta particle and a gamma ray. Write the equation for the transmutation reaction. 11. What fraction of sodium-24 would be left after 30 hours if the half-life of Na-24 is 15 hours? 12. Explain why radioactive uranium is found in nature. Why has it not all decayed into other elements? (Questions 13-15) Describe the change in mass number and atomic number when an atom emits: 13. an alpha particle 14. a beta particle 15. a gamma ray (Questions 16-17) A radioactive element, A, with a mass of 214 and atomic number 84, emits an alpha particle and changes to element B. Element B emits a beta particle and is converted to element C. 16. Write nuclear reactions equations for these elements. 17. Name the elements A, B, and C. 18. The half-life of I-131 is 8.07 days. How much will remain of a 50.0 microgram sample after 150 days?