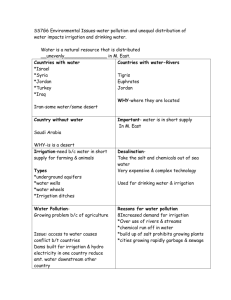
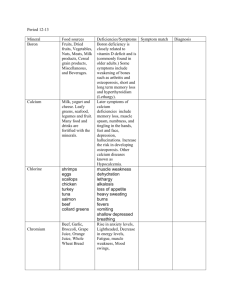
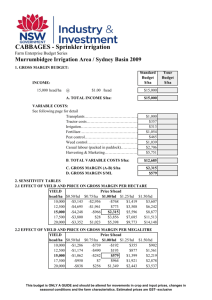
Table itemizing and offering highlights of studies addressing risk
advertisement

RISK ASSESSMENT OF LEAFY GREENS (last updated 1/10/2014) Reference Balbus, J., R. Parkin, and M. Embrey. 2000. Susceptibility in microbial risk assessment: Definitions and research needs. Environ. Hlth. Perspectives 108:901-905. Barker, S.F., J. O'Toole, M.I. Sinclair, K. Leder, M. Malawaraarachichi, and A.J. Hamilton. 2013. A probabilistic model of norovirus disease burden associated with greywater irrigation of home-produced lettuce in Melbourne, Australia. Wat. Res. 47:1421-1432. Carrasco, E., F. Pérez-Rodríguez, A. Valero, R.M. García-Gimeno, and G. Zurera. 2010. Risk assessment and management of Listeria monocytogenes in ready-to-eat lettuce salads. Comp. Rev. Food Sci. Food Safety 9:498. Coleman, E., K. Delea, K. Everstine,D. Reimann, D. Ripley, and the Environmental Health Specialists Network Working Group. 2013. Handling practices of fresh leafy greens in restaurants: Receiving and training. J. Food Prot. 76:2126-2131. da Cruz, A.G., S.A. Cenci, and M.C.A. Maia. 2006. Quality assurance requirements in produce processing. Trends Food Sci. Technol. 17:406-411. Danyluk, M.D. and D.W. Schaffner. 2011. Quantitative assessment of the microbial risk of leafy greens from farm to consumption: Preliminary framework, data, and risk estimates. J. Food Prot. 74:700-708. Ding, T., J. Iwahori, F. Kasuga, J. Wang, F. Forghani, M.-S. Park, and D.-H. Oh. 2013. Risk assessment for Listeria monocytogenes on lettuce from farm to table in Korea. Food Control 30:190-199. Notes Participants of a workshop acknowledged that a full consensus on how to define and incorporate susceptibility into microbial risk assessment was unlikely to emerge. Key conceptual issues included clarifying the distinction between individual- and population-scale definitions of susceptibility; identifying which intrinsic and extrinsic factors are modifiable and which health outcomes should be considered adverse; determining whether susceptibility exists in the absence of exposure or is conditional on it; and determining whether agent, exposure, or dose should be included in a definition of susceptibility. More than a quarter of greywater users in Australia irrigate their vegetable gardens with this source despite government advice against this practice. The annual disease burdens attributed to greywater irrigation ranged from 2 x 10-8 to 5 x 10-4, depending on the source of greywater and the existence of produce washing within households. The estimated norovirus disease burden associated with greywater irrigation of vegetables was estimated to be negligible relative to household contact with an infected individual. Food chain was modeled from processing of raw material at the factory up to consumption. Monte Carlo simulations of the model were run to estimate the number of cases in low-risk and high-risk populations. From the 4 risk management measures simulated and the goal of obtaining 100 cfu/g throughout the shelf-life of lettuce, the injection of a mixture of gases into packages at manufacture was the most effective in reducing the number of cases, followed by 4 d of storage at home, and prevention of high-risk consumers from consumption of ready-to-eat lettuce. The survey revealed that appropriate handling procedures assist in the mitigation of other unsafe handling practices for leafy greens. This review contains information on the main factors responsible for the elaboration of a quality assurance system for produce plants: good agricultural practices (GAP) and good manufacturing practices (GMP), including the sanitation standard operating procedures (SSOP) and hazard analysis and critical control points (HACCP). This QMRA model represents a preliminary framework that identifies available data and important data gaps and provides initial risk estimates for pathogenic E. coli in leafy greens. Critical data gaps remain, which include estimates of initial pathogen prevalence and level in the field, the number of days that the product remains in the field after contamination, and the fraction of incoming product that may contain the pathogen. Research-based time range estimates for retail storage and correlations between time and temperature during storage are needed; and the importance of lag time in modeling E. coli O157:H7 growth in leafy greens is unknown. The model also predicts that a majority of simulated cases arise from leafy greens cross-contaminated during the washing process, which is based on extrapolation from a single study and requires additional validation. The whole food chain of lettuce from farm to table including initial contamination on the farm, growth and cross contamination during transportation, storage and consumption was simulated employing @Risk software and the results showed mean final contamination levels of -1.50 log CFU/g and -0.146 log CFU/g by L. monocytogenes at restaurant and home, respectively. Based on the model, the incidence of listeriosis ranged from 11.9 to 17.4 cases per million individuals. The QRAM in this study was calculated assuming the maximum-dose level as 7.5 log CFU/serving. Compiled by Marilyn Erickson, Center for Food Safety, University of Georgia Downloaded from the website: A Systems Approach for Produce Safety: A Research Project Addressing Leafy Greens found at: http://www.ugacfs.org/producesafety/index.html. See http://www.ugacfs.org/producesafety/Pages/TermsofUse.html for disclaimers & terms for use of information in this document. Page 1 RISK ASSESSMENT OF LEAFY GREENS (last updated 1/10/2014) Reference Franz, E., A.V. Semenov, and A.H.C. van Bruggen. 2008. Modelling the contamination of lettuce with Escherichia coli O157:H7 from manure-amended soil and the effect of intervention strategies. J. Appl. Microbiol. 105:1569-1584. Franz, E., S.O. Tromp, H. Rijgersberg, and H.J. van der Fels-Klerx. 2010. Quantitative microbial risk assessment for Escherichia coli O157:H7, Salmonella, and Listeria monocytogenes in leafy green vegetables consumed at salad bars. J. Food Prot. 73:274-285. Gale, P. 2004. Risks to farm animals from pathogens in composted catering waste containing meat. Vet. Rec. 155:77-82. Gale, P. 2005. Land application of treated sewage sludge: quantifying pathogen risks from consumption of crops. J. Appl. Microbiol. 98:380396. Gale, P. and G. Stanfield. 2001. Towards a quantitative risk assessment for BSE in sewage sludge. J. Appl. Microbiol. 91:563569. Notes The model estimated an average of 0.34 contaminated heads per hectare. A minimum storage time of 30 days and a minimium fertilization-to-planting interval of 60 days was most successful in reducing the risk. Sensitivity analysis revealed that the likelihood of contamination was most sensitive to the prevalence of contaminated manure, the manure storage time and the initial density of E. coli O157:H7 in naturally contaminated manure. Increasing the manure storage time (to a minimum of 30 days) and incorporating a fertilization-to-planting interval of at least 60 days were most successful in reducing the number of contaminated lettuce heads. This study included an integration of modeling pathogen growth in the supply chain of fresh leafy vegetables destined for restaurant salad bars. Did not account for any lag phase in pathogen growth. Temperature in cold chain was considered to be 2-5°C whereas at salad bar, temperatures ranged from 0-13°C. As a result, growth of E. coli O157:H7 and Salmonella was minimal (17 and 15%, respectively) while growth of L. monocytogenes was considerably greater (194%). Because of the low virulence of this latter pathogen, it was not considered problematic. The estimated number of annual infection cases was considered reasonable in relation to epidemiological data (42 to 551 cases for O157; 81 to 281 for Salmonella; and 0.1 to 0.9 for L. monocytogenes). The factors controlling the level of risk are the separation of the meat at source, the efficiency of the composting process, and the decay and dilution of the pathogens in soil. The net pathogen destruction by the composting process is determined largely by the degree of bypass. Targeted 7 pathogens (Listeria monocytogenes, Campylobacters, E. coli O157, C. parvum, Giardia, and enteroviruses) on root crops. Using laboratory data for pathogen destruction by mesophilic anaerobic digestion, and not extrapolating experimental data for pathogen decay in soil to the full 30-month harvest interval specified by the Matrix, predicts 50 Giardia infections per year, but less than one infection per year for the other 6 pathogens. Assuming linear decay in the soil, a 12- month harvest interval eliminates the risks from all 7 pathogens; the highest predicted being one infection of C. parvum in the UK every 45 years. Lack of knowledge on the exact nature of soil decay processes is a source of uncertainty. The main sources of uncertainty in the risk assessment are the degree to which sewage sludge treatment destroys BSE agent, whether there is a threshold dose for initiation of BSE infection in cattle, and most importantly, the amount of brain and spinal cord material which enters the sewer from the abattoir. The model developed in this paper suggests that recycling of BSE agent through sewage sludge will not sustain endemic levels of BSE in the UK cattle herd. The risks to humans through consumption of vegetable crops are acceptably low. Compiled by Marilyn Erickson, Center for Food Safety, University of Georgia Downloaded from the website: A Systems Approach for Produce Safety: A Research Project Addressing Leafy Greens found at: http://www.ugacfs.org/producesafety/index.html. See http://www.ugacfs.org/producesafety/Pages/TermsofUse.html for disclaimers & terms for use of information in this document. Page 2 RISK ASSESSMENT OF LEAFY GREENS (last updated 1/10/2014) Reference Hamilton, A.J., F. Stagnitti, R. Premier, A.-M. Boland, andn G. Hale. 2006. Quantitative microbial risk assessment models for consumption of raw vegetables irrigated with reclaimed water. Appl. Environ. Microbiol. 72:3284-3290. Harrison, J.A., J.W. Gaskin, M.A. Harrison, J.L. Cannon, R.R. Boyer, and G.W. Zehnder. 2013. Survey of food safety practices on small to medium-sized farms and in farmers markets. J. Food Prot. 76:1989-1993. Hoelzer, K., R. Poulillot, K. Egan, and S. Dennis. 2012. Produce consumption in the United States: An analysis of consumption frequencies, serving sizes, processing forms, and highconsuming population subgroups for microbial risk assessments. J. Food Prot. 75:328-340. Holley, R.A. 2011. Food safety challenges within North American Free Trade Agreement (NAFTA) partners. Compr. Rev. Food Sci. Food Safety 10:131. Notes The models presented cover what would generally be considered worst case scenarios: overhead irrigation and consumption of raw vegetables. Models were run for several different scenarios of crop type, viral concentration in effluent, and time since last irrigation. Necessary data on the volume of irrigation water captured by these crops was estimated in a field study. In determining the likely concentration of enteric viruses on the product, adopted the previously used conservative approach whereby it was assumed that all pathogens in the irrigation water found on the plant attached to it. The annual risk of infection ranged from 10-3 to 10-1 when reclaimed-water irrigation ceased 1 day before harvest and from 10-9 to 10-3 when it ceased 2 weeks before harvest. Two previously published decay coefficients were used to describe the die-off of viruses in the environment. For all combinations of crop type and effluent quality, application of the more aggressive decay coefficient led to annual risks of infection that satisfied the commonly propounded benchmark of <10-4, i.e., one infection or less per 10,000 people per year, providing that 14 days had elapsed since irrigation with reclaimed water. Conversely, this benchmark was not attained for any combination of crop and water quality when this withholding period was 1 day. The lower decay rate conferred markedly less protection, with broccoli and cucumber being the only crops satisfying the 10-4 standard for all water qualities after a 14-day withholding period. The mean annual risk of infection was always less for cucumber than for broccoli, cabbage, or lettuce. Variation in the amount of produce consumed had the most significant effect on the total uncertainty surrounding the estimate of annual infection risk. Survey data was collected from 226 farmers and 45 market managers. More than 56% of farmers use manures with 34% of those using raw or mixtures of raw and composted manure, and over 26% wait fewer than 90 days between application of raw manure and harvest. Water that has not been tested for safety for irrigation is used by over 27% of farmers while 16% use such water sources for washing produce. Surfaces that touch produce at the farm is not sanitized by over 43% of farmers while 67% of farmers do not clean transport containers between uses. Food safety standards for over 42% of farmers markets are absent according to responses by market managers and only 2 to 11% of these managers ask farmers specific questions about conditions on the farm that could affect product safety. Market surfaces are sanitized by less than 25% of managers and even fewer managers (11%) clean market containers between uses. Less than 25% of managers offer sanitation training to workers or vendors. Data showed that produce consumption differs among fruits and vegetables, fresh and heat-treated foods, and demographic groups. Such results are valuable for risk assessments and allow targeting of risk communication or interventions to those individuals at greatest risk. Foodborne illness surveillance and reporting are most comprehensive in the U.S., but it is uniformly more reactive than proactive in all 3 countries. Food Safety policy is based on outbreak data, but that may be short-sighted because they represent roughly 10% of foodborne illness cases. Compiled by Marilyn Erickson, Center for Food Safety, University of Georgia Downloaded from the website: A Systems Approach for Produce Safety: A Research Project Addressing Leafy Greens found at: http://www.ugacfs.org/producesafety/index.html. See http://www.ugacfs.org/producesafety/Pages/TermsofUse.html for disclaimers & terms for use of information in this document. Page 3 RISK ASSESSMENT OF LEAFY GREENS (last updated 1/10/2014) Reference EPA and USDA Interagency Microbiological Risk Assessment Guideline Workgroup. 2012. Microbial risk assessment guideline. Pathogenic microorganisms with focus on food and water. Available at: http://www.fsis.usda.gov/PDF/Microbi al_Risk_Assessment_Guideline_2012001.pdf. Accessed 8/13/2012. Jacxsens, L., P.A. Luning, J.G.A.J. van der Vorst, F. Devlieghere, R. Leemans, and M. Uyttendaele. 2010. Simulation modeling and risk assessment as tools to identify the impact of climate change on microbiological food safety – The case study of fresh produce supply chain. Food Res. Int. 43:1925-1935. Kirezieva, K., J. Nanyunja, L. Jacxsens, J.G.A.J. van der Vorst, ,M. Uyttendaele, and P.A. Luning. 2013. Context factors affecting design and operation of food safety management systems in the fresh produce chain. Trends Food Sci. Technol. 32:108-127. Leifert, C., K. Ball, N. Volakakis, and J.M. Cooper. 2008. Control of enteric pathogens in ready-to-eat vegetable crops in organic and ‘low input’ production systems: a HACCPbased approach. J. Appl. Microbiol. 105:931-950. McKellar, R.C., D.I. LeBlanc, F.P. Rodríguez, and P. Delaquis. 2013. Comparative simulation of Escherichia coli O157:H7 behaviour in packaged fresh-cut lettuce distributed in a typical Canadian supply chain in the summer and winter. Food Control 35:192-199. Mena, K.D. and S.D. Pillai. 2008. An approach for developing quantitative risk-based microbial standards for fresh produce. J. Water Hlth. 6:359-364. Notes The goal of the 231-page document is to produce more consistent and transparent microbial risk assessments across participating federal agencies. It addresses the entire risk assessment process from an introduction to terminology and roles of the participants to planning the risk assessment, identifying and characterizing the hazard, assessing how the size of an outbreak may be affected by the dose (exposure assessment) or how the severity of the disease may be affected by the pathogen and its response within the human hose (dose-response assessment). It also provides information about microbial risk management and risk communication. The proposed knowledge-based modeling system is a most appropriate way to identify impacts of anticipated climate change and globalization on microbiological food safety of fresh produce. Next to this, additional research will be needed towards technological pre- and post-harvest solutions (e.g. growing and irrigation techniques, water treatment techniques) in order to be able to control the food safety of fresh produce. The major context factors that create risk to decision-making in food safety management systems in fresh produce chain were defined in this study. In addition, a tool for their systematic analysis was developed. This review describes 6 Risk Reduction Points (RRPs) where risks from enteric pathogens can be reduced in ready-to-eat vegetables. Changes can be made to animal husbandry practices (RRP1) to reduce inoculum levels in manure. Outdoor livestock management (RRP2) can be optimized to eliminate the risk of faecal material entering irrigation water. Manure storage and processing (RRP3), soil management practices (RRP4) and timing of manure application (RRP5), can be adjusted to reduce the survival of pathogens originating from manure. During irrigation (RRP6), pathogen risks can be reduced by choosing a clean water source and minimizing the chances of faecal material splashing on to the crop. Reported on temperature profiles measured in winter and summer months in a retail supply chain and their predicted impact on the fate of E. coli O157:H7 in fresh-cut lettuce using the stochastic simulation model, @RISK. Outputs from the model demonstrated a range of possible outcomes for the time-temperature profiles collected from the commercial supply chain and ranged from slight growth to die-off. Risks of infection are estimated using typical monitoring data of Salmonella detected on carrots and assuming various scenarios of the likelihood of an individual consuming a contaminated serving of carrots in a given year. Estimated annual risks of infection range from 2.20 x 10-5 to 2.16 x 10-3, assuming 1 % and 100% of an individual’s carrot servings are contaminated, respectively. Compiled by Marilyn Erickson, Center for Food Safety, University of Georgia Downloaded from the website: A Systems Approach for Produce Safety: A Research Project Addressing Leafy Greens found at: http://www.ugacfs.org/producesafety/index.html. See http://www.ugacfs.org/producesafety/Pages/TermsofUse.html for disclaimers & terms for use of information in this document. Page 4 RISK ASSESSMENT OF LEAFY GREENS (last updated 1/10/2014) Reference Mota, A., K.D. Mena, M. SotoBeltran, P.M. Tarwater, and C. Cháidez. 2009. Risk assessment of Cryptosporidium and Giardia in water irrigating fresh produce in Mexico. J. Food Prot. 72:2184-2188. Mukherjee, A., D. Speh, and F. DiezGonzalez. 2007. Association of farm management practices with risk of Escherichia coli contamination in pre-harvest produce grown in Minnesota and Wisconsin. Int. J. Food Microbiol. 120:296-302. Nabulo, G., S.D. Young, and C.R. Black. 2010. Assessing risk to human health from tropical leafy vegetables grown on contaminated urban soils. Sci. Total Environ. 408:5338-5351. OMAF Food Inspection Branch. 2001. Carrot risk assessment introduction and summary. http://www.omafra.gov.on.ca/english/f ood/inspection/fruitveg/risk_assessme nt_pdf/carrot/30ra.pdf. Pérez Rodríguez, F., D. Campos, E.T. Ryser, A.L. Buchholz, G.D. PosadaIzauierdo, B.P. Marks, G. Zurera, and E. Todd. 2011. A mathematical risk model for Escherichia coli O157:H7 cross-contamination of lettuce during processing. Food Microbiol. 28:694-701. Petterson, S.R., N.J. Ashbolt, and A. Sharma. 2001. Microbial risks from wastewater irrigation of salad crops: A screening-level risk assessment. Wat. Environ. Res. 73:667-672. Puerto-Gomez, A.F., J. Kim, R.G. Moreira, G.-A. Klutke, and M.E. Castell-Perez. 2013. Quantitative assessment of the effectiveness of intervention steps to reduce the risk of contamination of ready-to-eat baby spinach with Salmonella. Food Control 31:410-418. Notes There have been limited studies regarding the volume of irrigation water retained on specific produce. As other risk modeling studies have done, used an estimated average of 0.0036 ml/g retained on cucumbers (i.e. smooth produce) and an estimated average of 0.108 ml/g water retained on lettuce (i.e. rough produce). Also assumed that all of the (oo)cysts detected in the irrigation water were transferred to the produce for a worst-case approach. Furthermore, also assumed that all detected (oo)cysts were infectious to humans. Am’t of produce consumed per person in the U.S. estimates were 13.0, 4.3, 3.3, and 6.2 g/day of tomatoes, bell peppers, cucumbers, and lettuce, respectively. Annual risks range from 9 x 106 for Cryptosporidium at the lowest concentration associated with bell peppers to almost 2 x 101 for exposure to Giardia on lettuce at the highest detected concentration. In Minnesota, surveyed 14 organic, 30 semi-organic, and 19 conventional farms. Approximately 44 to 55% of conventional farms used animal waste as fertilizer, while 70 to 100% of the semi-organic and organic farms had animal manure as fertilizer. The use of animal wastes for fertilization of produce plants increased the risk of E. coli contamination in organic and semi-organic produce significantly. Improper ageing of untreated animal manure significantly increased this risk in organic produce grown using such manure as a fertilizer. focus is on chemical contaminants There is some potential for biological hazards to contaminate carrots from preproduction to the retail level of trade, although the overall food safety risk was considered to be quite low. The greatest risk for carrots occurs during the handling, washing, grading, and packing processes and at the retail level of trade, where carrots are sold as hand-harvested bunched carrots, or loose in bulk displays. A probabilistic model was constructed to account for E. coli O157:H7 cross contamination when contaminated lettuce enters the processing line. Three different scenarios were considered to represent the initial concentration on the contaminated batch entering the processing line (0.01, 1 and 100 cfu/g). The initial concentration in the contaminated batch did not influence significantly the pathogen levels in bags derived from cross contamination however prevalence levels were impacted. At the lowest contamination level, prevalence levels were predicted to be less than 1%. In contrast, prevalence levels of 3 and 13% were predicted at the higher initial contamination levels of 1 and 100 cfu/g, respectively. The model showed that the pathogen was able to survive and be present in the final bags in all simulated interventions scenarios although irradiation (0.5 kGy) was a more effective decontamination step in reducing prevalence than chlorination or pathogen testing. Predicted infection rates were much more sensitive to the decay rate of viruses than occasional high virus numbers. It was assumed that any microorganism contained in the residual wastewater remaining on the irrigated crop would cling to the leaf even after the wastewater itself evaporated. Intervention strategies (temperature control during harvest, washing, and irradiation) was integrated into the risk assessment model. Based on a low level of crosscontamination of bacteria (1 log CFU/g), the percentage of samples over the safety limit (1.33 log CFU/g sampl) was estimated at 16.8% but increased to 84% if a high level of cross-contamination occurred (~3 log CFU/g). Even with this high number of tainted lots, exposure of the leafy greens to irradiation (1 kGy) would reduce the incidence to 0.1%. Compiled by Marilyn Erickson, Center for Food Safety, University of Georgia Downloaded from the website: A Systems Approach for Produce Safety: A Research Project Addressing Leafy Greens found at: http://www.ugacfs.org/producesafety/index.html. See http://www.ugacfs.org/producesafety/Pages/TermsofUse.html for disclaimers & terms for use of information in this document. Page 5 RISK ASSESSMENT OF LEAFY GREENS (last updated 1/10/2014) Reference Shuval, H., Y. Lampert, and B. Fattal. 1997. Development of a risk assessment approach for evaluating wastewater reuse standards for agriculture. Wat. Sci. Technol. 35(11-12):15-20. Signorini, M.L., M.V. Zbrun, A. Romero-Scharpen, C. Olivero, F. Bongiovanni, L.P. Soto, L.S. Frizzo, and M.R. Rosmini. 2013. Quantitative risk assessment of human campylobacteriosis by consumption of salad crosscontaminated with thermophilic Campylobacter spp. from broiler meat in Argentina. Prev. Vet. Med. 109:37-46. Stine, S.W., I. Song, C.Y. Choi, and C.P. Gerba. 2005. Application of microbial risk assessment to the development of standards for enteric pathogens in water used to irrigate fresh produce. J. Food Prot. 68:913-918. Strawn, L.K., Y.T. Gröhn, S. Warchocki, R.W. Worobo, E.A. Bihn, and M. Wiedmann. 2013. Risk factors associated with Salmonella and Listeria monocytogenes contamination of produce fields. Appl. Environ. Microbiol. 79:76187627. Notes Using the risk assessment model in this paper, it was determined that when irrigating ready-to-eat crops with wastewater effluent meeting the WHO guidelines (1,000 fecal coliforms/100 ml), the annual risk of contracting a virus disease was about 10 -6 to 10-7 and for rotavirus disease, it was about 10-5 to 10-6. Based on the statement by the U.S. EPA that guidelines for drinking water standards should be designed to ensure that human populations not be subjected to the risk of infection by enteric disease > 10 -4 for a yearly exposure, this study suggests that the WHO guidelines provide a factor of safety some 1-2 orders of magnitude greater than that called for by the U.S. EPA for microbial standards for drinking water. It was also estimated that expenditures of some $3 – 30 million dollars per case of disease prevented would be necessary to meet the standards for drinking water. The model prediction for risk of infection was variable according to the dose-response model. The risk of human campylobacteriosis was most sensitive to the probability of infection from a Campylobacter, followed by the number of Campylobacter spp. per serving, the frequency of washing the cutting board, the preparation of raw poultry before salad using the same cutting board, and the frequency of hand washing. The concentration of hepatitis A virus (HAV) and Salmonella in irrigation water (furrow or drip) necessary to achieve a 1:10,000 annual risk of infection from cantaloupes, iceberg lettuce, and bell peppers was calculated. These calculations were based on the transfer of the selected nonpathogenic surrogates to fresh produce via irrigation water, as well as previously determined preharvest inactivation rates of pathogenic microorganisms on the surfaces of fresh produce. The risk of infection was found to be variable depending on the type of crop, irrigation method, and days between last irrigation event and harvest. The worst-case scenario, in which produce is harvested and consumed the day after the last irrigation event and maximum exposure is assumed, indicated that concentrations of 2.5 CFU/100 of Salmonella and 2.5 x 10-5 MPN/100 ml of HAV in irrigation water would result in an annual risk of 1:10,000 when the crop was consumed. If 14 days elapsed before harvest, allowing for die-off of the pathogens, the concentrations were increased to 5.7 x 103 Salmonella/100 ml and 9.9 x 10-3 HAV/100 ml. Detection of Salmonella and Listeria monocytogenes occurred in 11% and 30% of water samples (n=74) and 6.1% and 17.5% of fields (n=263), respectively. Pathogenpositive water samples were primarily from nonirrigation surface water sources. A nanagement practice that increased the odds of a Salmonella-positive field included manure application within a year while the presence of a buffer zone had a protective effect. The likelihood of detecting a L. monocytogenes-positive field was increased if irrigation occurred within 3 days of sample collection, wildlife were observed within 3 days of sample collection, or the soil had been cultivated within 7 days of sample collection. Compiled by Marilyn Erickson, Center for Food Safety, University of Georgia Downloaded from the website: A Systems Approach for Produce Safety: A Research Project Addressing Leafy Greens found at: http://www.ugacfs.org/producesafety/index.html. See http://www.ugacfs.org/producesafety/Pages/TermsofUse.html for disclaimers & terms for use of information in this document. Page 6 RISK ASSESSMENT OF LEAFY GREENS (last updated 1/10/2014) Reference Szabo, E.A., L. Simons, M.J. Coventry, and M.B. Cole. 2003. Assessment of control measures to achieve a food safety objective of less than 100 cfu of Listeria monocytogenes per gram at the point of consumption for fresh precut iceberg lettuce. J. Food Prot. 66:256-264. Topp, E., A. Scott, D.R. Lapen, E. Lyautey, and P. Duriez. 2009. Livestock waste treatment systems for reducing environmental exposure to hazardous enteric pathogens: Some considerations. Bioresource Technol. 100:5395-5398. Tromp, S.O., H. Rijgersberg, and E. Franz. 2010. Quantitative microbial risk assessement for Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes in leafy green vegetables consumed at salad bars, based on modeling supply chain logistics. J. Food Prot. 73:1830-1840. Walls, I. 2007. Framework for identification and collection of data useful for risk assessments of microbial foodborne or waterborne hazards: A report from the International Life Sciences Institute Research Foundation Advisory Committee on data collection for microbial risk assessment. J. Food Prot. 70:1744-1751. Watanabe, T., D. Sano, and T. Omura. 2002. Risk evaluation for pathogenic bacteria and viruses in sewage sludge compost. Wat. Sci. Technol. 46(11-12):325-330. Notes The food safety objective (FSO) offers an approach to translate public health risk into a definable goal wherein there is a specified maximum frequency or concentration of a hazardous agent in a food at the time of consumption that is deemed acceptable to provide an appropriate level of health protection. In the case of L. monocytogenes, there is a proposed FSO of <100 cfu/g in RTE products. A FAO/WHO panel calculated that 100% compliance for this goal would lead to approximately 5 to 25 cases of listeriosis per year, or a 99% reduction from the current baseline. As an example of how this concept would work, if the increase in concentration due to growth of viable L. monocytogenes remaining after washing was assumed to be as high as 2.7 log CFU/g and the initial level of contamination on whole lettuce were as high as 0.1 log MPN/g, then a performance criterion of a 0.8 log reduction is required to meet the FSO. The beneficial impacts of livestock waste treatment on risk to humans via exposure to manured land are illustrated using quantitative microbial risk assessment scenarios. Assumed geometric mean pathogen concentrations of 20 Cryptosporidium oocysts/g and 320 Campylobacter cells/g fresh weight cattle manure. Human exposure is assumed to result from ingestion of soil shortly after application of manure (25 tonnes/hectare), and hence little opportunity for die-off in the soil. In the absence of livestock waste treatment, the risk of infection (expressed as a probability of risk of infection per exposure event) from Cryptosporidium was 1.75 x 10-4, and from Campylobacter 1.27 x 10-2. In contrast, when considering treated livestock waste that had a 3 log reduction in pathogen content, the risk from Cryptosporidium was 1.75 x 10-7, and from Campylobacter was 1.27 x 10-5. The aim of this study was to quantitatively assess the difference between simulating supply chain logistics (MOD, complete with storage delays) and assuming fixed storage times (FIX) in microbial risk estimation for the supply chain of fresh-cut leafy green vegetables destined for salad bars. The public health effects were assessed by conducting an exposure assessment and risk characterization. The relative growths of E. coli O157 (17%) and Salmonella enterica (15%) were identical in the MOD and FIX models. In contrast, the relative growth of Listeria monocytogenes was considerably higher in the MOD model than in the FIX model and consequently the risk of infection was higher in the MOD model than in the FIX model for this pathogen. The key data needs identified for a microbial risk assessment were as follows: (i) burden of foodborne or waterborne disease; (ii) microbial contamination of foods; and (iii) consumption patterns. In addition, dose-response data may be necessary, if existing dose-response data cannot be used to estimate dose response for the population of interest. In this study, several kinds of compost were investigated for detection of pathogenic bacteria (Salmonella spp. and E. coli O157) and enteric viruses. It was concluded from the result that these bacteria and viruses could not be detected in 1.0 g wet weight of any kinds of compost. Criteria satisfying the acceptable risk (less than 10 -4 per year) for these pathogenic bacteria and virus in the compost were determined from the result of simulations. 1.0 [CFU or PFU/gww] was available as the criteria for E. coli O157 and poliovirus 1 in the compost. On the other hand, the criterion for Salmonella spp. in the compost should be established on a lower concentration than 0.001 CFU/gww. Compiled by Marilyn Erickson, Center for Food Safety, University of Georgia Downloaded from the website: A Systems Approach for Produce Safety: A Research Project Addressing Leafy Greens found at: http://www.ugacfs.org/producesafety/index.html. See http://www.ugacfs.org/producesafety/Pages/TermsofUse.html for disclaimers & terms for use of information in this document. Page 7