SNC 2D- CHEMISTRY INTRO QUIZ
advertisement
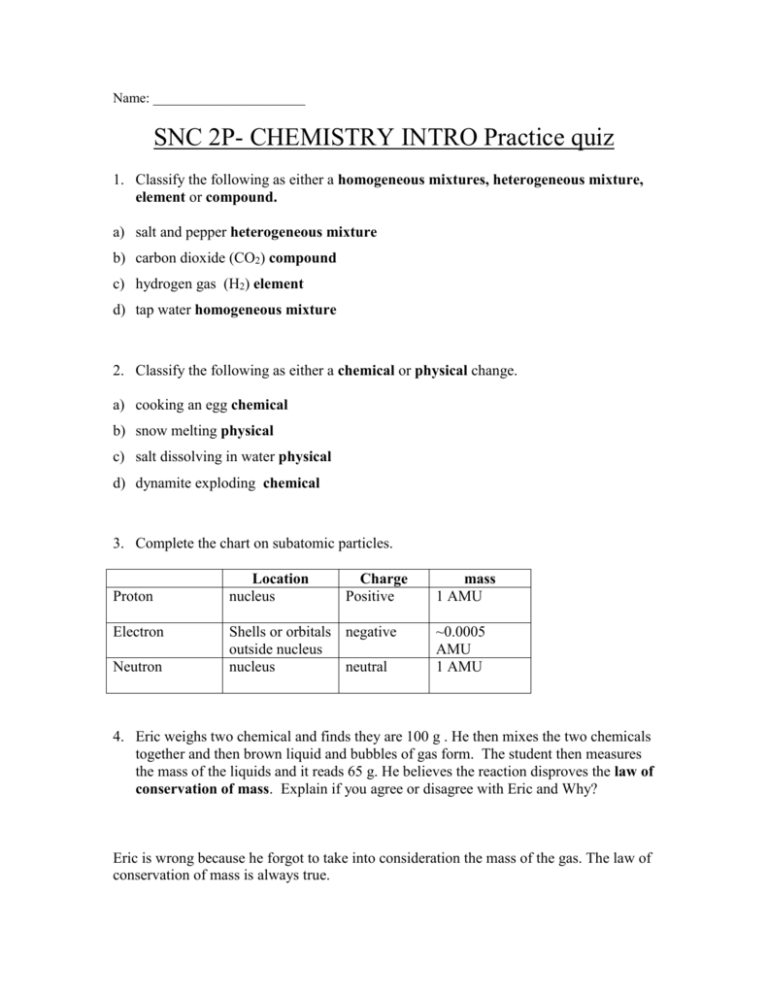
Name: ______________________ SNC 2P- CHEMISTRY INTRO Practice quiz 1. Classify the following as either a homogeneous mixtures, heterogeneous mixture, element or compound. a) salt and pepper heterogeneous mixture b) carbon dioxide (CO2) compound c) hydrogen gas (H2) element d) tap water homogeneous mixture 2. Classify the following as either a chemical or physical change. a) cooking an egg chemical b) snow melting physical c) salt dissolving in water physical d) dynamite exploding chemical 3. Complete the chart on subatomic particles. Proton Electron Neutron Location nucleus Charge Positive Shells or orbitals negative outside nucleus nucleus neutral mass 1 AMU ~0.0005 AMU 1 AMU 4. Eric weighs two chemical and finds they are 100 g . He then mixes the two chemicals together and then brown liquid and bubbles of gas form. The student then measures the mass of the liquids and it reads 65 g. He believes the reaction disproves the law of conservation of mass. Explain if you agree or disagree with Eric and Why? Eric is wrong because he forgot to take into consideration the mass of the gas. The law of conservation of mass is always true. 5a) Draw a Bohr diagram for oxygen b) Draw a Bohr diagram for an oxygen ion (oxygen in its stable form) c) What is the charge on an oxygen ion 2- d) Would the oxygen ion be a cation or an anion? anion











