Making Aspirin - Chemical Paradigms
advertisement

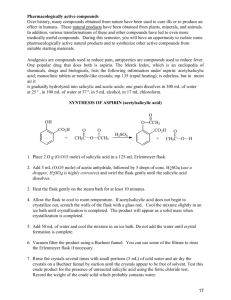
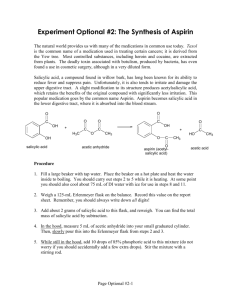
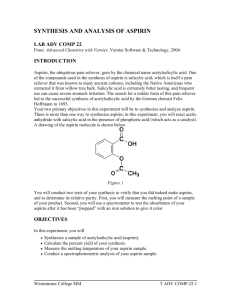
Making Aspirin Whitney Wu & Helen Yu Background Information Aspirin is an example of organic chemistry Functions as an analgesic (painkiller) and an antipyretic (reduces fever) 5th B.C. - Greek physician Hippocrates found that extract of willow tree bark, Salicin, be used to reduce fevers. 1829 - Salicin was isolated from willow bark and used as a pain reliever 1897 - German chemist, Felix Hoffman, was looking for a less acidic pain reliever which led to the synthesis of acetylsalicylic acid (ASA) or aspirin. Aspirin can be made by the simple one step chemical process by reacting salicylic acid with acetyl chloride, according to the reaction: Research Question How is aspirin synthesized? And what are the reactions that occur in the synthesis of aspirin? Outline of Methodology Making Aspirin 1. 1. 2. 3. 4. 5. 6. 7. 8. Place around 3 grams of salicylic acid in a 125-mL Erlenmeyer flask. Record the mass of salicylic acid used. In the fume hood, CAUTIOUSLY add 6-mL of acetic anhydride and 5 drops of concentrated sulfuric acid. Thoroughly mix reagents by swirling flask. Place flask in a beaker of water at a temperature of 80oC to 90oC and heat for 20 minutes. From the Erlenmeyer flask and allow to cool to room temperature. Add 40-mL of distilled water to the mixture in the Erlenmeyer flask and thoroughly mix the content by swirling. Cool the mixture in an ice bath to complete crystallization. Pour the mixture through filter paper place in an another Erlenmeyer flask and allow the crystals to air dry. Testing the Purity of Synthesized Aspirin 2. 1. 2. Dissolve a few crystals of salicylic acid and a few crystals of your aspiring in 5-mL of water in 3 separate test tubes. Add a drop of 1% iron chloride (FeCl3) to each test tube and note the color. (If you do not have 1% FeCl3 you can create it by adding 1 gram of FeCl3 to 100-mL of distilled water). Re-crystallization 3. 1. 2. 3. Dissolve about 6 grams in around 20-mL of absolute ethanol (100% ethanol) in a 125mL Erlenmeyer flask and warm in a hot water bath. Once dissolved add 50-mL of warm distilled water, after it has cooled filter the crystals. Once dry, test the re-crystallized material with 1% FeCl3 solution or impurities. Summary of Results M : 138.13 g/mol M : 180.17 g/mol m : 3.000 g m: 3.070 g n (salicylic acid): m (aspirin): Percentage Yield: Conclusion After we conducted our experiment and synthesized aspirin, we tested out the purity of it and our results showed that … There was Salicylic Acid contamination in our aspirin, tested positive due to the purple coloration in the FeCl3 test. 2) Sulfuric Acid was the catalyst in this reaction 3) In order for these reactions to occur: 1) Evaluation Our percentage yield for this experiment was 78.46%. Some probable reasons for our errors: There were still crystals left on the side of the Erlenmeyer flask. During our ice bath, our flask accidently fell completely into the water, adding more than 40-mL of distilled water. When we tested the purity of the aspirin, the instructions said to “add a few crystals” of aspirin and salicylic acid. This was vague thus not giving us a specific amount, varying the color spectrum of our test tubes. When the crystals of ASA were being filtered, some of it could have been still in liquid form as we poured it through the filter paper, thus being lost in the solution. Bibliography Jordan, Carol. "D.3 Analgesics." IB Chemistry Medicines and Drugs.Web. 19 May 2010. <http:// ibchemistrymedicinesanddrugs.wikispaces.com/D.3+Analgesics>. Pandita, Sangeeta, and Samta Goyal. Journal of Chemical Education.Vol. 75. 1998. Print. Ser. 6.