01g
advertisement

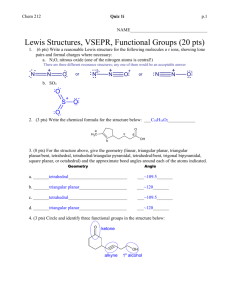
Chem 212 Quiz 1g p.1 NAME__________________________________ Lewis Str., VSEPR, Functional Groups (20 pts) 1. (9 pts) Each of the following molecules is a pollutant in our environment. Draw one Lewis structure for the each, showing lone pairs and formal charges where necessary: a. Ozone O3 (one of the oxygen atoms is the central atom) There are two equivalent resonance structures; either one is an acceptable answer : - O: O O : : or : : O : : : + : + O : - O : b. Dinitrogen monoxide N2O (one of the nitrogen atoms is the central atom) There are three different resonance structures; any one of them would be an acceptable answer O + or N + N - : : : : : : : + N 2- N : or : : O O : + N : N c. Sulfate anion, SO42Sulfur (and phosphorus, and others in the third row or below of the periodic table) can expand their octet. - : : : : : O O O : : S - : : O: 2. (3 pts) The structure below is related to a promising new class of anti-cancer drugs under clinical investigation. What is the chemical formula for this molecule? a alkyne OH secondary alcohol d b C10H8O2 O alkene ketone c 3. (4 pts) For the structure in question 2, use the VSEPR method to predict both the geometry (linear, triangular planar, triangular planar/bent, tetrahedral, tetrahedral/triangular pyramidal, tetrahedral/bent, octahedral) and bond angles around the atoms indicated by the arrows. a. tetrahedral/bent, 104º (don’t forget the two lone pairs on oxygen!) b. tetrahedral, 109.5º (don’t forget the two H’s on carbon!) c. linear, 180º d. triangular planar, 120º (don’t forget the H on the carbon!) 5. (4 pts) Circle and name three different functional groups in the structure in question 2. see above for the answer