Supplemental Questions for Chapters 1 & 3
advertisement

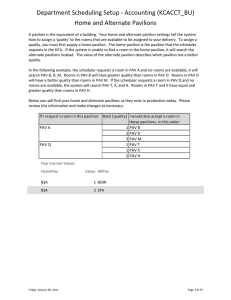
Chem 311 Pav 2012 Chapter 1 + 2: “Chemistry, Matter, and Change” Objectives The objectives are a list of items that you should be able to do upon completion of a unit. Although it would be helpful to create a written study guide from these questions, you are not required to do. You are, however, required to do the written work listed at the bottom of this page and the supplemental questions on the subsequent pages. Section 1.1 Chemistry (pg. 7-11) 1. Define Chemistry and describe its five major divisions. 3. Distinguish between pure and applied science. Be able to discuss examples of each. 4. Evaluate the impact chemists have on everyday life (on your own) Section 1.2 Chemistry Far and Wide (pg. 12-17) 1. Discuss the role of chemistry in the following areas: Materials, Energy, Medicine and Biotechnology, Agriculture, The Environment, and the Universe(on your own) Section 1.3 Thinking Like a Scientist (pg. 20-25) 1. Explain the relationship among the terms: Observations, experiment, hypothesis, theory and law in the scientific method. 2. Distinguish between independent and dependent variables, controls and constants. 3. Compare and contrast theories and laws in scientific knowledge. 4. Describe how scientific knowledge is shared and communicated (on your own). Section 2.1 Properties of Matter (pg. 39-42) 1. 2. 3. 4. Describe matter using extensive and intensive properties. Distinguish matter as a mixture or a substance. Name and characterize the three states of matter. Identify physical changes of matter. Section 2.2 Properties of Matter (pg. 44-47) 1. Classify a sample of matter as homogeneous or heterogeneous. 2. Describe the following separation techniques: Filtration, distillation, crystallization and chromatography(not in text) 3. Distinguish what types of mixtures can be separated by the techniques listed above Section 2.3 Elements and Compounds (pg. 48-52) 1. Explain the difference between an element and a compound. 2. Memorize the names and symbols of several elements. (See Mrs. Pav’s wikispace for list) Section 2.4 Chemical Reactions (pg. 53-55) 1. Compare physical and chemical changes in matter. 2. Identify the indications of a chemical change 3. State and apply the law of conservation of mass and atoms. Written Work These are selected problems from the end of the chapters being studied. To get credit for mathematical problems, you must show your solutions. Although not necessary, it is recommended that you copy the problem over again so that you can use your work as a study guide. These questions/problems are due at the beginning of the class period, the day of each test throughout the year. Randomly selected questions will be graded for accuracy. It is recommended that you attempt them by the DAY PRIOR TO THE TEST so that you can ask questions.) Chapter 1: p. 34-36 # 34, 36, 54, 62, 67, 68, 70, 74, 77, 78, 81 p. 37 # 2-14 even Chapter 2: p. 58-60 # 42, 45, 46, 47, 49, 52, 54, 56, 57, 60-63, 64, 66, 67, 69, 71, 76, 77 p. 61 # 2-14 even Chem 311 Pav 2012 Supplemental Questions for Chapters 1 & 3 These questions are assigned at the start of each unit and are meant to be completed as a student progresses through the content of each unit. The answers to these questions can be written in the spaces provided and are expected to be completed one day prior to a unit test. The unit packet will be collected at the beginning of the class period on the day prior to a unit test, checked for completion, and returned to a student before they leave class that day. 1. List the 5 branches of chemistry and provide a real-world example of work that is done in each branch. 2. Conduct an internet search on Benjamin Franklin’s “kite and key” experiment. Is this an example of pure or applied research? Justify your answer with an explanation. 3. You want to study the effect that sunlight has on plant growth. a. Write a hypothesis for your study using a “If,then” statement. b. What are your dependent variables and independent variables? c. What would be your constant variables? d. What would you use as a control? 4. What is the main difference between a theory and a law? Chem 311 Pav 2012 5. Classify a substances boiling point as an intensive or extensive property of matter. Explain. 6. Using the diagram below, answer the questions to the right. Which of the blocks (A-T) represent the following: 1. A homogeneous mixture of elements in a solid state? 2. A pure substance in the gaseous phase? 3. A heterogeneous mixture of compounds in the liquid state? 4. Which of the following represent a chemical change? (Select all that apply) P to D O to A B to C S to E 7. Does the number of phases present in a mixture indicate the number of components of that mixture? Explain. Chem 311 Pav 2012 8. Give a “real life” example of mixtures that could be separated using the following techniques and what the individual components would be following separation. Technique Mixture Name Components following Separation Filtration Distillation Crystallization Chromatography 9. Watch the youtube video entitled “Soda Musical”(http://www.youtube.com/watch?v=nrCaONL0X9U) and classify the change that you see as either physical or chemical. Explain your choice. 10. The Law of Conservation of Mass state that in a chemical reaction mass is neither created nor destroyed. Does the following demonstrate the Law of Conservation of Mass? Explain. 2KHCO3 + 2H3PO4 K2HPO4 + 4H2O + 2CO2