Specific Heat Capacity Worksheet: Physics Problems
advertisement

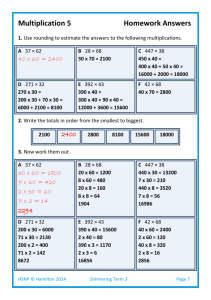
Name: _____________________________ Date: _____________ Assignment #1 Specific Heat Capacity Use ∆E = mc∆T to solve the following problems. 1. How much heat is needed to raise the temperature of 90.0 kg of water from 18˚C to 80°C? ∆E = mc∆T ∆E = (90.0 kg)(4200 J/kg/˚C)(80-18 ˚C) ∆E = 23 436 000 J = 23.4 MJ 2. If 1.0 MJ (megajoule) of heat is transferred to 10.0 kg of water initially at 15˚C, what will its final temperature be? 23.8˚C + 15˚C = 38.8˚C 3. If 12.0 kg of water cools from 100°C down to room temperature (20°C), how much heat will it release to the environment? ∆E = mc∆T ∆E = (12.0 kg)(4200 J/kg/˚C)(20-100 ˚C) ∆E = -4 032 000 J = -4.032 MJ (negatives indicates energy given off) 4. Why is water such a desirable material to use as a coolant in a car engine? Water has a very high heat capacity so it can hold a lot of heat, helping to cool down an engine more quickly. 5. If it takes 1200 J to raise the temperature of 0.500 kg of brass from 20.0˚C to 26.2°C, what is the specific heat capacity of brass? ∆E = mc∆T c = ∆E /(m∆T) c = (1200 J)/[(0.500kg)(26.2-20.0 ˚C)] c = 387 J/kg/˚C 6. How much heat would be needed to warm 1.6 kg of ice from -15°C up to its melting point of 0.0°C? ∆E = mc∆T ∆E = (1.6 kg)(2100 J/kg/˚C)(0.0- -15 ˚C) ∆E = 50 400 J = 50.4 kJ 7. A 5.0 kg block of lead at 250°C cools down to 20°C. How much heat does it give off in doing so? ∆E = mc∆T ∆E = (5.0 kg)(130 J/kg/˚C)(20-250 ˚C) ∆E = -149 500 J = -149.5 kJ (negative implies energy given off) Table of Specific Heat Capacities SUBSTANCE (J/kg/˚C) SUBSTANCE (J/kg/˚C) water 4200 steam 2100 methyl alcohol 2400 aluminum 920 ethylene glycol (antifreeze) 2200 glass 840 ice 2100 iron 450 kerosene 2100 copper 430 lead 130