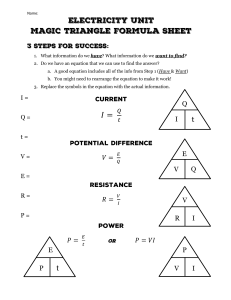
Title: Specific Heat Capacity Starter: 1. What happens when you bite into a McDonalds hot apple pie? 2. Explain why Challenge : Can you explain this in terms of energy in the particles? LO: What is Specific Heat Capacity and what can it tell us? Bronze: Explain Specific Heat Capacity in terms of the energy of particles. Silver: Calculate Specific Heat Capacity in familiar and unfamiliar systems. Gold: Carry out calculations involving rearranging the Specific Heat Capacity equation. LO: What is Specific Heat Capacity and what can it tell us? At the end of a sunny day at the beach, what do you notice about the temperature of the sand and the water? WHY??? LO: What is Specific Heat Capacity and what can it tell us? SAME amount of HEAT ENERGY Small TEMPERATURE RISE WATER Large TEMPERATURE RISE SAND LO: What is Specific Heat Capacity and what can it tell us? To increase the temperature of 1 kg of water by 1°C, requires 4200 J. To increase the temperature of 1 kg of copper by 1°C, requires 390 J. Different materials require different amounts of energy to heat up because they have different values for a property called specific heat capacity. The specific heat capacity for water is 4200 J/kg°C and for copper is 390 J/kg°C. LO: What is Specific Heat Capacity and what can it tell us? The amount of energy required to heat up a substance will depend on: 1) How much of it there is (mass) 2) How much you want to heat it up by (change in temperature) 3) The property of the material – this is called the specific heat capacity Key definition: Specific heat capacity is the amount of energy required to increase the temperature of 1 kg of a material by 1°C. The specific heat capacity for water is 4200 J/kg°C and for copper is 390 J/kg°C. Key words: 1kg, energy, material LO: What is Specific Heat Capacity and what can it tell us? The amount of energy required to heat up a substance will depend on: 1) How much of it there is (mass) 2) How much you want to heat it up by (change in temperature) 3) The property of the material – this is called the specific heat capacity Key definition: Specific heat capacity is the amount of energy required to increase the temperature of 1 kg of a material by 1°C. The specific heat capacity for water is 4200 J/kg°C and for copper is 390 J/kg°C. LO: What is Specific Heat Capacity and what can it tell us? Here is the formula we need to know: E=mxcxθ Greek symbol called theta Energy Mass x Specific Heat x Temperature = Capacity (____) change(__) Transferred (_) (__) Add in the Units: oC, kg, J, J/kgoC You WILL be given this formula Challenge: What do you notice about the unit for Specific Heat Capacity? LO: What is Specific Heat Capacity and what can it tell us? e.g. A kettle contains 1.5kg of water at a temperature of 18oC. How much energy is needed to bring the water to the boil? (specific heat capacity of water is 4200J/kgoC) m = 1.5kg E c = 4200J/kgoC = m xc xθ = 1.5 x 4200 x 18 = 113400 J θ = 100-18 = 82oC E = ? LO: What is Specific Heat Capacity and what can it tell us? Rearrange the specific heat capacity formula so that m is the subject E=mxcxθ LO: What is Specific Heat Capacity and what can it tell us? Rearrange the specific heat capacity formula so that c is the subject E=mxcxθ LO: What is Specific Heat Capacity and what can it tell us? Rearrange the specific heat capacity formula so that θ is the subject E=mxcxθ LO: What is Specific Heat Capacity and what can it tell us? How have you found today’s lesson? I need some extra help Try this sheet I have understood everything Try this sheet Plenary 1. What are the units for the following: • Mass • Energy • Specific heat capacity 2. What does theta represent? 3. Which one of has a high specific heat capacity, water or sand?