Calorimetry - Berkeley Cosmology Group
advertisement

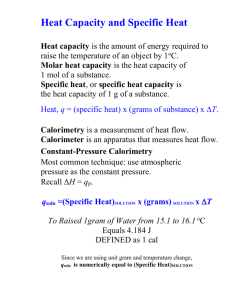
Topics in Science Research Project Leader: Bruno Serfass Members: Perry Francois-Edwards Jose Martinez Kadeem Palacios Matthew Constantino Calorimetry Our overall project objective was to study how we use calorimetry to detect the energy that a dark matter particle would deposit inside our calorimeter. We used ordinary particles from a radioactive source to demonstrate this measurement. The existence of dark matter can be proven by looking at objects such as stars or galaxies revolving around certain areas. The content that we can detect in these areas have far too little mass to create gravity that would make objects revolve around it. We can assume that these areas have extra matter that provides the needed gravity: dark matter. It is “dark” because it is matter that we cannot see or detect with the technology that we currently have. In order to understand how to detect dark matter, we must understand calorimetry. During the last two and one half weeks, we learned about heat capacity, heat conductivity, and calorimetry. Heat capacity is the ratio of heat absorbed by a substance to the substance’s increase in temperature. Heat conductivity is the rate at which thermal (heat) energy that can flow through a substance at a higher or lower temperature. Calorimetry is the measurement of the amount of heat absorbed in a chemical reaction, change of state, or form of a solution. We conducted four experiments to understand the concepts of heat capacity, heat conductivity, and calorimetry. First Experiment The first experiment we performed involved the concept of heat conductivity. The purpose of this experiment is to test and find out which metal out of those available would conduct heat most effectively. The following metals were used in this experiment: Copper, Aluminum, Brass, and Lead. Each rod of metal conducts heat at a different rate. We used a Bunsen burner as a steam boiler to heat the bottom of the rods at the same time at the same rate. We tracked the temperature by hooking up thermometers on the top of the rods and connected them to a laptop that makes a spreadsheet telling us the difference in temperature. The copper’s temperature raised highest and fastest which means that the heat moved to the top faster than all of the other metals. This shows that the copper had the highest heat conductivity. Second Experiment The second experiment we did involved using the concept of heat capacity. Heat capacity is the mass times the specific heat, which is defined as the amount of heat needed to change 1 gram of a substance’s temperature by 1°C. The purpose of this experiment is to test which of the five materials has the highest heat capacity. The five substances, of the same volume, we tested on were lead, glass, zinc, brass and iron. The way this experiment works is we heat up all test substances to 100°C. Then we release the balls onto a sheet of paraffin. Whichever substance melts through first has the most heat capacity. Each ball has a different mass and specific heat so therefore each have a different heat capacity. When we dropped the balls on the paraffin, we noticed that iron made it through the paraffin first. Zinc and brass made it halfway. The rest of the metals barely melted any of the paraffin at all. From this, we drew the conclusion that iron had the highest heat capacity out of those substances tested. One thing to note is that some balls had high mass but low specific heats so they didn’t fall through and vice versa. Third Experiment In another experiment, we used a small-scale calorimeter. A calorimeter isolates an object from emitting any heat to other non-measurable sources such as the air. The object is submerged in isolated water as to allow the heat to be transferred there. The temperature of the water is now measured as to see how much energy was transferred from the object to the water. The objective for this experiment was to find the energy that was transferred into the brass based on the change in temperature of the water by using this formula: q= mc(T2-T1) q is the energy that is being transferred m is the substance’s mass c is the specific heat which is the amount of heat needed to change the substance’s temperature by 1 degree T2-T1 is the change in temperature A calorimeter is shaped like a large can filled with water and layered with a type of pottery material that keeps heat from being transferred from the water inside to any outside source. The object we were trying to measure is a brass cylinder. The brass was suspended on a string and submerged in the water inside the calorimeter, not touching the inside walls of the calorimeter. We tried to measure and compare the temperature of the brass and only the water the brass was touching. However this proved too difficult considering the size of the brass compared to the area in which it was placed. The thermometer for the water only had touched both the brass and the inside of the calorimeter. Its not supposed to do that. Unfortunately there was no way we could accurately do this and this experiment was deemed unsuccessful. Fourth Experiment The formula above can be rearranged to show what the change in temperature depends on: T2-T1= q/mc When we tried to find the change in temperature involving a particle with very low energy (10 keV), we found the change way too small to be accurately measured. Thus the only way you can make the measurement more reasonable is to cool the substance down to a very low temperature. When you lower the temperature, you lower the specific heat (c). The specific heat is one of the dividers besides mass (m). If you lower the divider, the quotient will be larger and can be more accurately measured. In our fourth and final experiment, we used a huge fridge that uses liquid helium and a mixture of He3 and He4 gas to lower the temperature of the calorimeter. The absorber of the calorimeter (a germanium crystal) is connected to the fridge using copper links, which as shown in the first experiment, has a high conductivity. The objective of this experiment is to record the energy that is transferred into the calorimeter by a particle when it collides with the germanium crystal. The substance that we are trying to measure is a small particle instead of brass. A radioactive source will shoot particles through the germanium crystal. The particles will collide with the atoms in the crystal. When they collide, they leave energy in the form of vibrations. Just like the water, the crystal absorbs the energy but his time as vibrations instead of heat. We measure these vibrations or pulses or events just like the change in temperature. The events are recorded in under a second since these pulses appear very rapidly. They go up and immediately go down because the entire area is being put at a very low temperature by the helium bath. The vibrations are slowed down immediately after occurring due to the low temperature. We hope to use calorimetry to find the energy of a dark matter particle when it hits a germanium crystal deep underground. It’s underground because it is mostly isolated from all other particles that won’t interfere with the germanium crystal.