SCIENCE 10 - MIDTERM REVIEW

Sc 10 Name: ______________
Blk: _____ Date: _________
SCIENCE 10 - MIDTERM REVIEW
Chemistry (Chapters 8-10)
Vocabulary: define the following….
1. Matter that is made up of indivisible particles _____________________________________
2. matter that is in only one phase ________________________________
3. matter that is pure but contains different elements _________________________________
4. subatomic particles located in the nucleus _________________, _______________________
5. Matter that has more than one phase; also known as a colloid ____________________________
6. A family of one _____________________________
7. the number of bonds with which an element can combine ______________________________
8. Bohr model diagrams show this (2 words) ______________________ ____________________
9. The octet rule means that _________________________________________________________
______________________________________________________________________________
10. Sodium, potassium, and lithium belong to this family _________________________________
11. Family with 2 valence electrons that gives them up to other elements _____________________
12. The outermost electrons of an atom ________________________
13. Type of reaction that gives off heat
14. Type of reaction that forms water in the products
________________________ generates electrical current in water
________________________
15. A material which is used for cleaning products, is slippery, usually containing OH- ions and
________________________
16. A material which can change colour when added to different pH materials _________________
17. Periodic Table arranged according to this value _________________ ________________
Short Answer:
1.
Complete the following
FAMILY DESCRIPTION EXAMPLES
OF ELEMENTS
Halogens
Alkali Metals
Alkali-Earth
Metals
Noble Gases
2.
Complete the following chart.
Substance Characteristics and Uses
Acids
Bases
Examples
Salts
3.
Complete the following chart.
Symbol Atomic # Atomic
Mass
Be
Cu
Na
Br
Sn
# protons # electrons # neutrons Combining
Capacity
4.
What is the differences between ionic bonding and covalent bonding? Give examples of the compounds they form.________________________________________________________________________
____________________________________________________________________________
____________________________________________________________________________
5.
Complete the following:
Type of Description of Reaction
Reaction
Endothermic
Exothermic
Synthesis
Decomposition
Single
Replacement
Double
Replacement
Neutralization
6.
Write the names/formulas of the following:
Example Chemical Equation a) sodium sulphide _______________________ b) CaSO c) copper (I) nitride _______________________ d) SnI
2 e) aluminum hydroxide ________________________ f) PF
3
7.
Balance the following
4
________________________
________________________
________________________ a) ____ Sn + ______ O
2
____ SnO
2 b) ____ Fe
2
O
3
+ _____ H
2
_____ Fe + _____H
2
O c) ____ H
2
S + _____ KOH
_____ K
2
S d) ____ Al + _____ H
2
SO
4
____ H
2
+ ____ H
2
+ _____ Al
0
2
(SO
4
)
3
Geology (Chapters 12-13)
Vocabulary: write the correct term for the following ….
1.
Rocks formed directly from solidified magma:
2.
Rocks formed from magma that cooled and crystallized
BENEATH the Earth’s surface:
3.
Rocks formed from magma that cooled and crystallized
ABOVE the Earth’s surface:
4.
The age of one event compared to another:
5.
Bits of rock blown out of a volcano:
6.
The process whereby the nuclei of radioactive parent isotopes break down:
______________________
______________________
______________________
______________________
______________________
______________________
7.
A mechanical instrument used to measure ground motion.
8.
The graphic record made by the instrument in #7
14.
The bending and tilting of rock layers:
15.
A secondary earthquake that occurs after the main shock:
______________________
9.
A rough, uneven break (crack) in a body of rock or material:
10.
Transportation of weathered rock fragments from one place to another:
______________________
11.
The point of origin of an earthquake.
12.
A location on the surface of the Earth directly above the point of origin of an earthquake:
______________________
______________________
______________________
13.
A crack in a body of rock along which there has been some movement: ______________________
______________________
______________________
16.
Two or more atoms of the same element that have the same number of protons, but different numbers of neutrons:
17.
The amount of time required for half of the nuclei in a radioactive sample to decay:
18.
Fossils useful for indicating a period of geological time:
______________________
______________________
______________________
19.
Rock composed of many tiny pieces of weathered rock:
20.
Molten rock found below the Earth’s surface:
______________________
______________________
21.
A large wave caused by an earthquake originating on the ocean floor: ______________________
22.
A scale of earthquake intensity based upon the observation of the effects produced by ground movement: ______________________
Short Answer:
1.
Describe the differences between P-waves and S-waves.
2.
List in order the six layers that make up the Earth and indicate what state (solid, liquid, gas) they are.
3.
Explain how a seismograph works. (Use a diagram and explain what each part does)
4.
Describe the process that causes the Earth’s tectonic plates to move.
5.
Give four characteristics of a good index fossil.
6.
What sort of things can be dated using Carbon-14?
7.
There are four principles used to determine the relative age of rock layers. Describe these four principles. a) b) c) d)
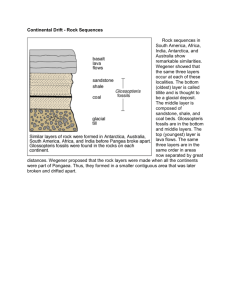
8.
Observe the diagram to the right and list the correct age sequence for the rock layers?
Youngest_______
_______
_______
_______
_______
Oldest_______
9.
Explain the theory behind radiometric dating.
10.
Plot a radioactive decay curve for uranium-235, which has a half-life of 713 million years.
(Time goes on the x-axis). Assume that you are starting with 400 mg of uranium-235.
Remember to label the axes. a) How many years will have passed by the time there is only about
1.5 mg of uranium left? ______________________
______________________ b) What is the decay product of uranium-235?