Appendix: Section 2.7 Substitution
Reactions of Alkanes
The AAMC released an updated MCAT® topic list following the publication of the
9th edition Examkrackers Manuals. The three pages that follow provide what you need
to know for substitution reactions of alkanes. Further updates to these Manuals based
on updates by the AAMC will be available to you on the Examkrackers Forums at
www.examkrackers.com.
PHOTOCOPYING & DISTRIBUTION POLICY
The illustrations and all other content in this book are copyrighted material owned by Osote
Publishing. Please do not reproduce any of the content, illustrations, charts, graphs, photos, etc.,
on email lists or websites.
Photocopying the pages so that the book can then be resold is a violation of copyright.
Schools and co-ops MAY NOT PHOTOCOPY any portion of this book. For more information, please
contact Osote Publishing: email: support@examkrackers.com or
phone 1.888.KRACKEM.
No part of this work may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, and recording, or by any information storage or retrieval system without prior written permission of
the copyright owner unless such copying is expressly permitted by federal copyright law, or unless it complies with the
Photocopying & Distribution Policy listed above.
© 2014 Osote Publishing
All rights reserved.
2.7
Substitution Reactions of Alkanes
[SN1 and SN2 reactions have been added to the AAMC MCAT® topic list since
the original publication of this manual. Further updates to these Manuals, including those based on AAMC updates to the MCAT® topic list, are available on the
Examkrackers Forums at www.examkrackers.com.]
Substitution reactions are fundamental reactions in organic chemistry.
In a substitution reaction one group leaves and is replaced with another. In
nucleophilic substitution, a leaving group is replaced by an incoming nucleophile.
Here, substitution reactions of alkanes will be considered. In the next lecture,
substitution reactions of carbonyls will be considered.
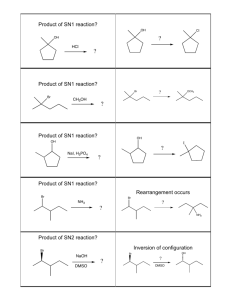
Substitution reactions occur when one functional group replaces another. Two
important types of substitution reactions are SN1 and SN2. These are substitution,
nucleophilic, unimolecular and bimolecular. The numbers represent the order of
the rate law and NOT the number of steps.
On the MCAT you will likely see
substitution reactions in the
context of alkyl alcohols – an alkane
with an alcohol – or in carbonyls.
Elimination reactions (E1 and E2)
are not tested.
SN1
An SN1 reaction has 2 steps and has a rate that is dependent on only one of the
reactants. The first step is the formation of the carbocation. This is the slow step
and thus the rate-determining step. Since this step has nothing to do with the
nucleophile, the rate is independent of the concentration of the nucleophile and
is directly proportional to the concentration of the substrate (the substrate is the
electrophile or the molecule being attacked by the nucleophile.) In an SN1 reaction
the leaving group (the group being replaced) simply breaks away on its own to
leave a carbocation behind. The second step happens very quickly. The nucleophile attacks the carbocation.
Notice that if the carbocation carbon began and ended an SN1 reaction as a chiral
carbon, both enantiomers would be produced. The intermediate carbocation is
planar and the nucleophile is able to attack it from either side. Carbon skeleton
rearrangement may occur if the carbocation can rearrange to a more stable form.
Elimination (E1) often accompanies SN1 reactions because the nucleophile may
act as a base to abstract a proton from the carbocation, forming a carbon-carbon
double bond.
FIGURE 2.25
Since the carbocation must be
formed spontaneously in an SN1
reaction, a tertiary substrate
is more likely to undergo an SN1
reaction than is a primary or
secondary substrate. On the MCAT,
probably only tertiary substrates
will undergo SN1. The rate of an SN1
reaction is determined solely by the
concentration of the substrate.
Mechanism of SN1 Reaction
Trigonal planar
carbocation
formed in step 1
CH₃
NaOH + H₃C
C
Cl
CH₃
Na+
Sodium ion
is non-reactive
Nucleophile attacks the
carbocation in step 2
CH₃
H2O
acetone
C+
H₃C
+
CH₃
OH–
H2O
acetone
CH₃
HO
C
CH₃
CH₃
Cl–
Halogen ion
spontaneously
drops off in step 1
LEC TURE 2: Introduction to Organic Chemistry
1
S N2
SN2 reactions occur in a single step. The rate is dependent on the concentration
of the nucleophile and the substrate. In an SN2 reaction a nucleophile attacks the
intact substrate from behind the leaving group and knocks the leaving group free
while bonding to the substrate.
FIGURE 2.26
Substitution, Nucleophilic, Bimolecular
H₃C
OH ⁻
C
H
CH₃
Br
HO
H
Br⁻
C
H
H
Inversion of configuration
Notice the inversion of configuration on the carbon being attacked by
the nucleophile. If the carbon were chiral, the relative configuration would be
changed but the absolute configuration might or might not be changed. Notice
also that a tertiary carbon would sterically hinder the nucleophile in this reaction. The rate of SN2 reactions decreases from primary to secondary substrates.
SN2 reactions don’t typically occur with tertiary substrates. If the nucleophile is
a strong base and the substrate is too hindered, an elimination (E2) reaction may
occur. In an E2 reaction, the nucleophile acts as a base abstracting a proton and,
in the same step, the halogen leaves the substrate forming a carbon-carbon double
bond. Bulky nucleophiles also hinder SN2 reactions.
Nucleophilicity
The strength of the nucleophile is unimportant for an SN1 reaction but important
for an SN2 reaction. A base is always a stronger nucleophile than its conjugate acid,
but basicity is not the same thing as nucleophilicity. If a nucleophile behaves as a
base, elimination results. To avoid this, we use a less bulky nucleophile. A negative charge and polarizability add to nucleophilicity. Electronegativity reduces
nucleophilicity. In general, nucleophilicity decreases going up and to the right on
the periodic table.
Leaving Groups
Consider relative nucleophilicity and
leaving group quality. The incoming
group must be more nucleophilic
than the leaving group. The outgoing
part of the molecule must have
better leaving group qualities than
the incoming piece. If the reverse
were true, the leaving group would
stay in place.
The best leaving groups are those that are stable when they leave. Generally speaking, the weaker the base, the better the leaving group. Electron withdrawing
effects and polarizability also make for a good leaving group. The leaving group
will always be more stable than the nucleophile.
Solvents
Polar protic solvents (polar solvents that can hydrogen bond) stabilize the nucleophile and any carbocation that may form. A stable nucleophile slows SN2 reactions,
while a stable carbocation increases the rate of SN1 reactions. Thus polar protic
solvents increase the rate of SN1 and decrease the rate of SN2. Polar aprotic solvents
(polar solvents that can’t form hydrogen bonds) do not form strong bonds with
ions and thus increase the rate of SN2 reactions while inhibiting SN1 reactions. In
SN1 reactions, the solvent is often heated to reflux (boiled) in order to provide
energy for the formation of the carbocation.
In solvolysis the solvent acts as the nucleophile.
2
E X A MKR ACKER S MCAT ® – CHEMISTRY
Solvation of the Nucleophile
FIGURE 2.28
H
OR
X:
–
H
OR
H
:
:O
O:
H
C
A solvated anion
(reduced nucleophilicity due to
enhanced ground-state stability)
O:
:O
:
H
O
:
H
H
H
:
H
H
+
:
OR
H
:
H
H
O
:
H
H
RO
Stabilization of the Carbocation Intermediate
:
FIGURE 2.27
H
SN1 vs. SN2
There are six things to remember about SN1 vs. SN2. Remember the six things as “The nucleophile and the five Ss”:
1) Substrate; 2) Solvent; 3) Speed; 4) Stereochemistry; and 5) Skeleton rearrangement.
The nucleophile: SN2 requires a strong nucleophile, while nucleophilic strength doesn’t affect SN1.
1st S:SN2 reactions don’t occur with a sterically hindered Substrate. SN2 requires a methyl, primary, or
secondary substrate, while SN1 requires a secondary or tertiary substrate.
2nd S:A highly polar Solvent increases the reaction rate of SN1 by stabilizing the carbocation, but slows
down SN2 reactions by stabilizing the nucleophile.
3rd S:The Speed of an SN2 reaction depends upon the concentration of the substrate and the nucleophile,
while the speed of an SN1 depends only on the substrate.
4th S:SN2 inverts Stereochemistry about the chiral center. An SN1 reaction creates both enantiomers.
5th S:SN1 may be accompanied by carbon Skeleton rearrangement, but SN2 never rearranges the carbon
skeleton.
Also remember that elimination reactions can accompany both SN1 and SN2 reactions. Substitution reactions can
also run in reverse. One example is when a halide is the nucleophile and a carbonyl is the leaving group. This is a good way
of synthesizing alkyl halides that could appear on the MCAT.
LEC TURE 2: Introduction to Organic Chemistry
3