Dendritic Spines and Memory
advertisement

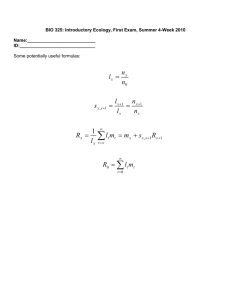
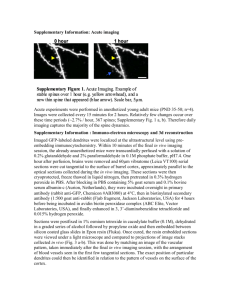
12/15/12 Ev ernote Web Dendritic Spines and Memory Saturday, December 15 2012, 12:01 PM Dendritic Spines and Memory Citation: Segal, M (2004) History of Neuroscience: Dendritic Spines and Memory, IBRO History of Neuroscience [http://www.ibro.info/Pub/Pub_Main_Display.asp?LC_Docs_ID=3534] Accessed: date Menahem Segal Soon after Ramon y Cajal first described the dendritic spine, over a hundred years ago, and while there was still a hot debate going on whether the neurons are isolated or are all linked in a continuous cytoplasmic net, Cajal and others had already proposed that the spine is the locus of long-term synaptic plasticity associated with storage of memories in the brain (Cajal, 1995). This intuitive dogma, which lasts to this day, stands on two pillars: the observation that spines vary tremendously in shape, size and density even on the same dendrite, and that this variability is correlated with the recent history of the organism; and that spines are formed during development, following the formation of dendrites, when experiences are stamped into the brain, and they disappear in aged animals, when old memories normally fade and new ones do not form. Enriched environment enhances them, and mentally retarded children express immature spines (Purpura, 1974; Scheibel et al., 1975; Greenough et al., 1987). Since excitatory synapses reside on spine heads, and synapses have always been assumed to change in process of learning, it was only natural to assume that spine shape/size/density is correlated with memory storage. But what exactly is the change that takes place in the spine head for it to 'store memories'? Obviously, these are extremely difficult questions to tackle, as one needs first to define 'a memory', then to find the neurons that store it, and to identify the dendritic location of the synapses that are involved in this particular memory, before asking what are the changes in these spines that can be considered as 'memory'. Basic questions such as Are different types of memories related to different morphologies of the spines? Do the spines change from one form to another once the 'memory' changes its strength/duration? Do memories make spines, or do spines allow memories to form? and corollary questions, e.g. Is size important? i.e. Do strong memories make larger spines, or do larger spines contain more/stronger memories? What happens to the spine once the memory fades/extinguishes? are only some of the plethora of questions that need to be addressed in a systematic manner before a causal relations between spines and specific function is to be assumed. Beyond that, it is not entirely clear that the postsynaptic structure, hosted by the dendritic spine, is actually the site of memory. Two other sites compete for seniority: there is a great deal of evidence to indicate that the presynaptic terminal is the one to be modified by a learning experience, at least at the initial stages of memory formation. In addition, postsynaptic, dendritic mechanisms, beyond the synapse, have also been implicated in long-term changes in neuronal interactions. Changes in brain morphology, found for example in animals raised in enriched https://www.ev ernote.com/edit/61ab5bf 3-e857-4d1f -88ae-7b508b3ce290#st=p&n=61ab5bf 3-e857-4d… 1/6 12/15/12 Ev ernote Web environment, are not restricted to the dendritic spine: the neocortex undergoes concomitant changes in thickness, vascularization, length and complexity of dendrites (Globus et al., 1973; Greenough et al., 1987). Formal attempts to correlate structural changes with functional ones involved application of a tetanic stimulation of a specific afferent pathway to the studied neurons, the production of longterm potentiation (LTP), followed by a search for changes in the spines of these neurons. Conducted both in vivo and in slice preparations, these studies did not yield a coherent view on what is actually the structural change in correlation with the functional changes: Reported changes range from swelling of the spine head (Van Harreveld and Fifkova, 1975), an increase in synaptic contact length (Lee et al. 1980; Desmond and Levy, 1983), spine head bifurcation (Comery et al., 1996; Rusakov et al., 1997), increases in spine density (Trommald et al., 1996), lack of changes in spine number and an increase in number of shaft synapses (Desmond and Levy, 1983, or presynaptic changes, expressed by a terminal touching more than one dendritic spine (Toni et al. 1999). This lack of coherent picture stems from the heterogeneity of cell type studied, type of stimulation employed and time after onset of the measured functional change, resolution of the methods of measurements, and methodological considerations. The intuitive assumption in these studies is 'the more the better', i.e. the more spines on a dendrite, the 'better' are its functions. Thus, if animals are raised in enriched environments, their spine density is higher and consequently their ability to learn more complex functions (see e.g. Moser et al. 1997). As a result, even increases in spine densities that could not be related to enriched life experience, e.g. those occurring across the estrus cycle in female rats (Woolley and McEwen, 1993), were attempted to be correlated with improved learning ability and enhanced expression of LTP of reactivity to afferent stimulation. In fact, if indeed there is any change in LTP expression across the estrus cycle, it is not nearly as dramatic as the change in spine density. Another, tangential, issue is that of spine pruning that may take place during maturation of neurons, alongside the formation of novel spines. Pruning of spines, like cell death, was assumed in the past to take place only during development or aging, but not as a result of an ongoing learning experience in the young adult. It was only recently, when spine pruning was also found to depend on activation of the NMDA receptor, that it was considered as a legitimate process that may lead to improved neuronal 'performance' (Bock and Braun, 1999), refuting the assumption that more is better. It was only recently, when spines could be visualized in living neurons using time-lapse photography both in vitro and in vivo (Segal, 1995; Yuste and Denk, 1995), that the research of spine structure and function began to gain momentum. Specifically, cultured neurons where spines could be visualized at high resolution for extended periods of time were found to be useful for this analysis. A radical change in the traditional view of the spine emerged; instead of a stable locus of synaptic interaction, the spine was now seen as a motile structure, which undergoes ongoing changes in shape and possibly in function on a minute-to-minute basis (Fischer et al., 1998; Halpain et al., 1998; Korkotian and Segal, 2001). These observations were confirmed in the in vivo brain using 2-photon microscopy, which allows the imaging of deep brain structures with an unprecedented resolution. The bridge between the emerging and the traditional views of spine plasticity remains a real challenge for future research. https://www.ev ernote.com/edit/61ab5bf 3-e857-4d1f -88ae-7b508b3ce290#st=p&n=61ab5bf 3-e857-4d… 2/6 12/15/12 Ev ernote Web Figure 1: Cultured hippocampal neurons express plastic, functional dendritic spines. A 2-week-old cultured neuron loaded with calcein was imaged at 3D in the confocal microscope to show a variety of spine shapes and sizes (A). The cell was exposed to FM4-64 dye that is taken up by active presynaptic terminals, and a section of the dendrite shown in A is magnified to demonstrate that the spines are touched by terminal boutons (B). Formation of a novel spine following a transient (5 minute) exposure of the culture to a conditioning medium that favors activation of the NMDA receptor (C). Images are taken before, left, and at 30-minute intervals after exposure to the conditioning medium. The total length of the spine is 1.5µm (modified from Goldin et al. 2001). The ability to time-lapse-photograph morphological variations in the same dendrite/spines over several hours reignited the search for spine-associated morphological basis of memory. In a pioneering study, Engert and Bonhoeffer (1999) described the formation of novel spines in a neuron undergoing long-term potentiation of reactivity to afferent stimulation. Novel spines were formed in response to plasticity-producing stimuli in a manner that was selective for the stimulated dendritic segment, and were not found in unstimulated segments of the dendritic tree of the same neuron. This indicates that an association has been formed between pre- and postsynaptic activity, as has been postulated for the 'Hebbian' synapse. It is now possible to begin to ask what is the nature of the stimuli that govern changes in spine morphology, and what are the functional consequences of such changes. Mechanisms for spine modification most likely involve a change in intracellular calcium concentration ([Ca2+]i). Early studies have shown that exposure of dendrites to high concentration of glutamate, resulting in a large and continuous rise in [Ca2+]i, causes shrinkage of the exposed spines, to their complete elimination (Segal, 1995c, Halpain et al. 1998). Exposure to stimuli that cause lower rises in [Ca]i resulted in elongation of existing spines, and occasionally also formation of novel ones (Korkotian and Segal, 1999). In fact, the same spine could be seen to shrink in response to high glutamate concentration, and to expand in response to low glutamate. The functional relevance of these morphological variations was indicated in studies that examined the spine/dendrite interaction, showing that longer spines are more detached functionally from the parent dendrite than short ones (Volfovsky et al., 1999). Further work on cultured pyramidal neurons using flash photolysis of caged glutamate has shown a distinct relationship between spine morphology and its ability to respond to glutamate: the larger spines appeared to be more affected by glutamate than the small ones, at least in as much as they were able to transmit the signal to the soma. This led to the suggestion that large spines are the backbone of functional synaptic connections, while the small thin ones are only on their way to maturation and are as yet functional (Kasai et al., 2003). Activation of the spines can convert them into functional ones (Matsuzaki et al., 2004). Thus, an understanding of the role of spine shape in integration of information is beginning to emerge, and there is evidence for all possible mechanisms for learning-related long-term changes in spines, including formation of novel spines, increase in spine head dimension as well as conversion of a silent to an active spine with no apparent change in morphology, but the molecular mechanisms responsible for these morphological changes in spines are far from being understood. https://www.ev ernote.com/edit/61ab5bf 3-e857-4d1f -88ae-7b508b3ce290#st=p&n=61ab5bf 3-e857-4d… 3/6 12/15/12 Ev ernote Web Still, it is not clear that the novel spines formed in the process of conditioning/LTP are at all functional, in that they contribute to the enhanced synaptic currents observed in the affected cells. Obviously, the novel synapses are formed 30-60 minutes after the establishment of LTP, indicating that they are not involved in short-term plasticity, but their role in the longer-term potentiation is still not clarified. While these observations are awaiting confirmation and extension by other research groups in brain slices, we (Goldin et al. 2001) have found that a transient exposure of dissociated tissue cultured hippocampal neurons to conditions that enhance activation of the NMDA receptor cause a sustained elevation of network activity, and can trigger the formation of novel dendritic spines. This enhanced activity is self-perpetuating and may promote a continuous change in network connectivity. These spines are likely to be functional, in that they are innervated by active presynaptic terminals, (Goldin et al. 2001). Concomitant with the formation of novel spines, there is a marked pruning of existing ones. The pruning of spines, originally thought to be present only in developing or dying neurons, has recently been found to be an active process, involving activation of the NMDA receptor (Bock and Braun, 1999). In our current studies, pruning has also been associated with activation of the NMDA receptor, and to correlate with an enhanced network activity. Unlike novel spines, pruned spines seem not to be associated with an active presynaptic terminal (Goldin et al., 2001), indicating that presence of an active terminal tends to enhance existing synapses, and its absence tends to cause elimination of probably non-functional dendritic spines. This dual behavior, the formation of spines in association with an active presynaptic terminal and the pruning of a non active synapse, can be considered as the morphological manifestation of the Hebbian rule, where binding of pre- and postsynaptic activity leads to growth of a synapse. Perspectives Current molecular and biophysical methods have rapidly expanded our knowledge of the composition of the spine to the extent that we now recognize more than 600 different molecules in this tiny organelle, and there is an emerging need to use system biological methods to place some order in this exceedingly complex structure. Still, the major issues are yet to be resolved: What are the changes that the spines undergo in process of learning that will make them the right candidates for storage of memory? How is a decision made to produce a spine in a certain place on the dendrite, and not on others? How is spine plasticity related to LTP or LTD? and What happens to new spines once the 'memory' is erased by a mechanism akin to LTD? These and similar issues await further refinement of research tools but, once available, they may bring us even closer to understanding the mechanisms that underlie the morphological and functional heterogeneity of spines, seen first over a hundred years ago, and that still constitute an enigma. The current ability to produce longer spines or spines with larger heads in a selective population of transfected neurons will undoubtedly contribute to the understanding the functional significance of spine heterogeneity and, through this, to the understanding of the role of spines in synaptic interaction and plasticity. Menahem Segal, PhD Department of Neurobiology The Weizmann Institute Rehovot 76100 Israel Phone: +972 89 342553 Fax: +972 89 344140 menahem.segal@weizmann.ac.il References Bock J., Braun K. (1999a) Blockade of N-methyl-D-aspartate receptor activation suppresses learning-induced synaptic elimination. Proc. Natl. Acad. Sci. USA, 96: 2485-90. Cajal Ramon y, Santiago (1995 English Translation) Histology of the Nervous System of Man and Vertebrates.New York: Oxford University Press. https://www.ev ernote.com/edit/61ab5bf 3-e857-4d1f -88ae-7b508b3ce290#st=p&n=61ab5bf 3-e857-4d… 4/6 12/15/12 Ev ernote Web Comery TA, Stamoudis CX, Irwin SA, Greenough WT.) 1996) Increased density of multiple-head dendritic spines on medium-sized spiny neurons of the striatum in rats reared in a complex environment. Neurobiol Learn Mem. 66(2):9 3-6. Desmond NL. and Levy WB. (1983) Synaptic correlates of associative potentiation/ depression: an ultrastructural study in the hippocampus. Brain Res. 265: 21-30. Engert F. Bonhoeffer T. (1999) Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature 399: 66-70. Fischer M Kaech S Knutti D and Matus A. (1998) Rapid actin based plasticity in dendritic spines. Neuron 20: 847-54. Globus A, Rosenzweig MR, Bennett EL, Diamond MC (1973) Effects of differential experience on dendritic spine counts in rat cerebral cortex. J. Comp. Physiol. Psychol. 82: 175-81. Goldin M. Segal M and E. Avignone (2001) Functional plasticity triggers formation and pruning of dendritic spines in cultured hippocampal networks. J. Neuroscience 21: 186-193. Greenough WT, Black JE, Wallace CS.) 1987) Experience and brain development. Child Dev. 58: 539-59. Halpain S. Hipolito A. and Saffer L. (1998) Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18:9835-9844. Kasai H Matsuzaki M Noguchi J Yasumatsu N and Nakahara H. (2003) Structure-stability-function relationships of dendritic spines. Trends in Neurosci. 26:360-8 Kim CH, Lisman JE (1999) A role of actin filament in synaptic transmission and long-term potentiation. J. Neurosci. 19:4314-24. Korkotian E. and Segal M.(1999) Release of calcium from stores alters the morphology of Dendritic spines in cultured hippocampal neurons. Proc. Nat. Acad. Sci. USA 96: 12068-72. Korkotian E. and Segal M (2001) Regulation of dendritic spine motility in cultured hippocampal neurons. J. Neuroscience 21: 6115-6124. Lee KS, Schottler F, Oliver M, Lynch G. (1980) Brief bursts of high-frequency stimulation produce two types of structural change in rat hippocampus. J. Neurophysiol. 44: 413-422. Matsuzaki M Honkura N Ellis-Davis GCR, and H. Kasai (2004) Structural basis of long term potentiation in single dendritic spines. Nature 429: XX-XX. Moser MB Trommald M. Egeland T and P. Andersen (1997) Spatial training in a complex environment and isolation alter the spine distribution differently in rat CA1 pyramidal cells. J. Comp. Neurol 380: 373-81. Purpura DP. (1974) Dendritic spine dysgenesis and mental retardation. Science 186:1126-8. Rusakov DA. Richter-Levin G, Stewart MG, Bliss TV. (1997) Reduction in spine density associated with long-term potentiation in the dentate gyrus suggests a spine fusion- and-branching model of potentiation. Hippocampus 7: 489-500. Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel A (1975) Progressive dendritic changes in aging human cortex. Exptl. Neurol. 47: 392-403. Segal M. (1995) Imaging of calcium variations in dendritic spines of cultured hippocampal neurons. J. Physiol. 486: 285-296. Toni N. Bucks PA, Nikohenko I. Bron CR and Muller D. (1999) LTP promotes formation of multiple https://www.ev ernote.com/edit/61ab5bf 3-e857-4d1f -88ae-7b508b3ce290#st=p&n=61ab5bf 3-e857-4d… 5/6 12/15/12 Ev ernote Web spine synapses between a single axon terminal and a dendrite. Nature 402, 421-425. Trommald M. Hulleberg G and P. Andersen (1996) Long term potentiation is associated with new excitatory spine synapses on rat dentate granule cells. Learning and Memory 3: 218-228. Van Harreveld A. and Fifkova E. (1975) Swelling of dendritic spines in the fascia dentata after stimulation of the perforant fibers as a mechanism of post-tetanic potentiation. Exptl. Neurol. 49: 736-749. Volfovsky N Parnas H Segal M and Korkotian E (1999) Geometry of dendritic spines affects calcium dynamics in hippocampal neurons: theory and experiments. J. Neurophysiol. 82: 450-462. Woolley CS and McEwen BS (1993) Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 336: 293-306. Yuste R. and Denk W (1995) Dendritic spines as basic functional units of neuronal integration. Nature 375: 682-684. https://www.ev ernote.com/edit/61ab5bf 3-e857-4d1f -88ae-7b508b3ce290#st=p&n=61ab5bf 3-e857-4d… 6/6