Leaching of Zinc Oxide in Acidic Solution
advertisement

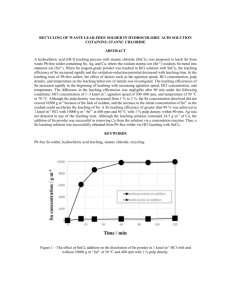
Materials Transactions, Vol. 44, No. 12 (2003) pp. 2489 to 2493 Special Issue on New Systems and Processes in Recycling and High Performance Waste Treatments #2003 The Japan Institute of Metals Leaching of Zinc Oxide in Acidic Solution Takashi Yoshida MS Zinc Co., Ltd., Tokyo 105-0004, Japan Leaching mechanism of zinc oxide is important not only to understand zinc smelting process but also to develop of new zinc recycling process. Recycling of zinc is mainly done by treatment of electric arc furnace dust (EAF dust). In Japan pyro metallurgical process is enhanced for EAF dust treatment, however only zinc oxide can be recovered by pyro metallurgical process. Hydro metallurgical process makes it possible to recover high purity zinc directly from EAF dust. One of the main components of zinc included in EAF dusts is zinc oxide. Thus the mechanism of zinc oxide leaching is important to develop the new hydro metallurgical zinc recycling process. Leaching test of zinc oxide in hydrochloric acid and sulfuric acid solution has been carried out kinetically. Zinc oxide disk was used for leaching test which the reaction area is clear. The experimental parameters of pH, temperature, leaching time and rotating speed of the disk specimen were varied. The results obtained are as follows; (1) The activation energy of leaching reaction by sulfuric acid was 17.5 kJmol1 and 11.6 kJmol1 by hydrochloric acid solutions. (2) The leaching rate increases in proportion to the square root of rotating speed of test specimen. These results indicate that the rate controlling factor of zinc oxide leaching reaction in acidic solution is mass transportation. (Received July 14, 2003; Accepted September 5, 2003) Keywords: leaching, zinc oxide, mass transfer, disk specimen, recycling and acid solution 1. Introduction The amount of zinc production in the world have increased and reached more than eight million tons. The major zinc production process is the hydro-metallurgical process which consists of roasting, leaching and electro-winning. The kinetics of leaching is very important to fully understand the zinc smelting process. Thus several studies1–4) have been done in the leaching kinetics of zinc oxide. On the other hand the recycling has become the major issue for almost every industry. The one of the major recycling of zinc is done by treatment of electric arc furnace dusts (EAF dust). Pyrometallurgical processes such as Wealz kiln process, MF process and electric thermal process are enhanced for recycling of zinc from EAF dust in Japan.5) These processes recover zinc as zinc oxide. Thus further treatment is necessary to produce high purity zinc metal. Hydro-metallurgical process makes it possible to produce high purity zinc directly from EAF dust. Several hydro-metallurgical processes have been proposed6) however they are not sufficient from the industrial point of view. Thus development of new hydro-metallurgical process for zinc recycling is very much concerned. Zinc included in EAF dust is mainly zinc oxide. Thus the basic study on leaching kinetics of zinc oxide becomes more important for development of new recycling process. Relatively many studies have been done for leaching kinetics of metal sulfide and oxide which controlled by chemical reaction.7–9) Few studies have been made on leaching kinetics which controlled by mass transportation. Leaching of zinc oxide by acidic solution is typical example that the reaction is controlled by mass transportation. Previous studies on leaching of zinc oxide in acidic solutions are carried out mainly by using powder samples. In these cases the surface area is not well understood. In this study disk type zinc oxide specimen is used to make sure the surface area and fully understand of leaching kinetics of zinc oxide. Furthermore the changing of surface morphologies was investigated. The observation of the surface morphologies is very few in previous studies. 2. Experimental Procedure 2.1 Samples Reagent grade zinc oxide powders are used for making zinc oxide disk. Zinc oxide powders were dried at 473 K for 7.2 ks then pressed under the pressure of 490 GPa. The pressed disk was sintered at 1523 K for 28.8 ks. The diameter of sintered disk is 10 103 m. The specific density of the disk was approximately 97 pct of the theoretical value. The disk specimen was coated in an epoxy resin. The one surface of the disk was exposed and polished by emery paper where leaching reaction occurred. Chemical reagent grade acids and deionized water was used for every leaching test. 2.2 Experimental apparatus for leaching test A glass flask containing 0.5 dm3 of leaching solution was used for reaction vessels of all leaching experiments. The disk specimen of zinc oxide attached to the rod and submerged to the leaching solution. The rod and the specimen were rotated by the variable rotating speed motor. The temperature was measured by thermocouple and controlled in the water bath. The temperature difference was controlled within 0:5 K during leaching experiments. Sulfuric acid and hydrochloric acid were used for leaching test. The pH value was maintained in same value during the leaching test because the volume of acid solution has large enough and the amount of dissolved zinc oxide was rather small. It was cleared by preliminary experiments to maintain pH value constant during the leaching tests. The samples of leaching solution were withdrawn periodically for quantitative analysis to determine the amounts of dissolved zinc by using ICP (Inductively coupled plasma) mass analyzer. The surface of zinc oxide disk specimen was observed before and after leaching tests by scanning electron microscope (SEM). The experimental conditions are summarized in Table 1. 2490 T. Yoshida Acid H2 SO4 , HCl Temperature (K) 303, 323, 333, 353 pH 1, 2, 3 Rotating speed of specimen (min1 ) 0, 100, 300, 1200 Experimental Results and Discussions 3.1 The morphology of the leaching tests Figure 1 shows the typical morphologies of test specimens observed by SEM. The surface morphology of the zinc oxide specimen before leaching test is almost flat and it is understandable because the specific density of the zinc oxide specimen was 97 pct of the theoretical value. After leaching tests, the surface morphologies changed that surface shown the bumpy shape in both hydrochloric acid and sulfuric acid leaching test. 3.2 Leaching curves at pH 1, 2 and 3. The leaching curves of zinc oxide disks, leached by hydrochloric and sulfuric acid at pH 1, 2 and 3 are illustrated in Figs. 2 and 3 respectively. In these figures C represents the amounts of dissolved zinc to the liquid phase. The leaching temperature was controlled at 333 K and the rotating speed of the disk specimen was 10 s1 . It is clear that the amounts of dissolved zinc increase according to the leaching time at each pH value and the leaching kinetics are linear for 7.2 ks. When the pH value was lower the amounts of dissolved zinc increased in both acids. Figure 4 illustrates the relationship between R (Leaching rate) and pH value in hydrochloric and sulfuric acid solutions. The kinetics of leaching rate shows linear relations with pH value. Hydrolytic reactions of Zn2þ hardly occur in the pH 1 to 3, thus pH value can be the parameter of aHþ (activity of hydrogen). So it can be said that the leaching kinetics of zinc oxide show linear relationship to activity of Hþ in the sulfuric acid and hydrochloric acid solutions when the pH value maintained 1 to 3. 3.3 Temperature dependence on leaching rate Temperature dependence on the leaching rate of zinc oxide was examined. The leaching curves when the leaching temperature was 303, 323, 333 and 353 K are shown in Figs. 5 and 6. Figure 5 shows the leaching curves in hydrochloric (a) 10 9 C / 10-3 mol . dm-2 3. Experimental conditions pH1 8 pH2 7 pH3 6 5 4 3 2 1 0 0 1200 2400 3600 4800 6000 7200 Leaching Time, t / s Fig. 2 Zinc oxide leaching curves in hydrochloric acid solutions at 333 K. The rotating speed was 10 s1 . 16 pH1 14 C / × 10-3 mol . dm-2 Table 1 pH2 12 pH3 10 8 6 4 2 0 0 1200 2400 3600 4880 6000 7200 Leaching Time, t / s Fig. 3 Zinc oxide leaching curves in sulfuric acid solutions at 333 K and the rotating speed of specimen was 10 s1 . acid solution at pH 2 and Fig. 6 shows the leaching curves in sulfuric acid at pH 2. The rotating speed of the specimen was controlled at 10 s1 in each experiment. The leaching kinetics (b) 50 µ m 50 µ m Fig. 1 Surface morphology of Zn oxide disk leached in hydrochloric acid solutions at 333 K. The rotating speed was 10 s1 : (a) before leaching (b)after leaching for 7.2 ks. Leaching of Zinc Oxide in Acidic Solution -5 H2SO4 353K 0.15 HCl 333K -6 323K C / 10-3 mol . dm-2 Log ( R / mol- dm-2 - s-1 ) 2491 -7 -8 303K 0.1 0.05 1 2 3 pH Fig. 4 Effect of pH value on the reaching rate of zinc oxide in hydrochloric acid and sulfuric acid at 363 K. Rotating speed of specimen was 10 s1 . 0 0 1200 2400 3600 4800 6000 7200 Leaching Time, t / s 1.0 Fig. 6 Leaching curves of zinc oxide in sulfuric acid solutions at pH 2 and rotating speed was 10 s1 . 353K 333K 323K 0.6 H2SO4 303K Ln ( R / mol . dm -2 . min-1 ) C / 10-3 mol . dm-2 0.8 0.4 0.2 HCl –12.5 17.5kJ . mol-1 –12 11.6kJ . mol-1 –11.5 0 0 1200 2400 3600 4800 6000 7200 2.9 Leaching Time, t / s Fig. 5 Leaching curves of zinc oxide in hydrochloric acid solutions at pH 2 and rotating speed was 10 s1 . of both leaching tests by hydrochloric acid and sulfuric acids in temperature range from 303 to 353 K are linear. The higher temperature enhanced the leaching volume in both acidic solutions. The data are summarized in the Arrhenius plot in Fig. 7. The leaching rates were determined by the gradient of the leaching curves of each leaching test of different temperature. The logarithmic value of the leaching rate and 1=T where T is the leaching temperature shows good linear relationship. The activation energy (Ea) can be expressed as follows; k ¼ k0 expðEa=RTÞ ð1Þ ln k ¼ ln k0 Ea=T ð2Þ In above equations k is the overall reaction coefficient and k0 is constant. Based on these equations the activation energy can be 3.0 3.1 3.2 3.3 Temperature T -1/ 10-3 K-1 Fig. 7 Arrhenius plot of zinc oxide leaching in hydrochloric acid and sulfuric acid solutions at pH 2 and rotating speed was 10 s1 . calculated by the gradient of each line in Fig. 7. The activation energy for leaching of zinc oxide disk by hydrochloric acid at pH2 is 11.6 kJmol1 and 17.5 kJmol1 by sulfuric acid solutions at the temperature range from 303 to 353 K. These activation energies show good agreement obtained by previous studies.1,4) Generally the activation energy of diffusion in liquid phase is less than 20 kJmol1 and the activation energy of chemical reaction is approximately 50 to 100 kJmol1 . These small values of the activation energy suggest the leaching rates of zinc oxide disks in hydrochloric acid and sulfuric acid solutions are controlled by mass transportation such as diffusion of chemicals through the liquid boundary layer. 2492 T. Yoshida 3.4 Effects of the rotating speed The leaching reaction of zinc oxide by hydrochloric acid and sulfuric acid solutions can be expressed as follows; ZnO þ H2 SO4 ¼ ZnSO4 þ H2 O ð4Þ The mechanism of leaching reaction can be describe that the diffusion of the chemical reagent to the surface of the zinc oxide disk through the liquid boundary layer and the leaching reaction occurs on the surface of the specimen. Then the products of the chemical reactions move from the surface to the leaching solution through the liquid boundary layer. Thus the kinetic of the leaching reaction is basically mixtures of chemical reactions and mass transportations. When the chemical reactions of eqs. (3) and (4) occur fast enough the leaching reaction is controlled by mass transportations. If leaching reactions of zinc oxide in hydrochloric acid and sulfuric acid solutions are controlled by the mass transportations the leaching rates should depend upon the rotating speed of the zinc oxide specimen. Basically the thickness of the liquid boundary layer on the disk specimen decreases when the rotating speed of the specimen increases. Therefore, to determine the rate controlled factor the effects of the rotating speed of the zinc oxide specimen on leaching reactions were carried out. The leaching curves of zinc oxide in different rotating speeds by hydrochloric acid solutions and sulfuric acid solutions are illustrated in Figs. 8 and 9 respectively. The rotating speed changed from 0 to 20 s1 and pH value was maintained at 2. The leaching temperature was 333 K in each test. The leaching kinetics are linear in each rotating speed of zinc oxide specimen. As illustrated in Figs. 8 and 9, the amounts of dissolved zinc oxide increased by leaching time and rotating speed as well. The thickness of liquid boundary layers decreases in proportion to the square root of the rotating speed of the 600 min-1 300 min-1 1.2 100 min-1 C / 10-3 mol . dm-2 ð3Þ 0 min-1 1.0 0.8 0.6 0.4 0.2 0 0 1200 2400 3600 4800 6000 7200 Leaching Time, t / s. Fig. 9 Leaching curves of zinc oxide in sulfuric acid solutions at pH 2 and temperature was 333 K. R / 10-6 mol - dm-2 - s-1 ZnO þ 2 HCl ¼ ZnCl2 þ H2 O 1200 min-1 1.4 0.2 H2SO4 HCl 0.15 0.1 0.05 0 01 1.0 1200 min-1 2 Rotating 3 Speed1/2, ω/s 4 1/2 600 min-1 300 min-1 C / 10-3 mol . dm-2 0.8 100 min-1 Fig. 10 Effect of rotating speed of specimen on zinc oxide leaching in hydrochloric acid and sulfuric acid solutions at the 333 K and the pH value was 2. 0 min-1 0.6 0.4 0.2 0 0 1200 2400 3600 4800 6000 7200 Leaching Time, t / s. Fig. 8 Leaching curves of zinc oxide in hydrochloric acid solutions at pH 2 and temperature was 333 K. specimen. The leaching rates of zinc oxide and square root of the rotating speed is illustrated in Fig. 10. The leaching rates show the linear relationship with square root of rotating speed in both hydrochloric acid and sulfuric acid where the rotating speed was between 0 to 20 s1 . It indicates that the leaching rates of zinc oxide disk increase with increasing rotating speed and these phenomena suggests the rate of zinc oxide leaching is controlled by diffusion through the liquid boundary layer. In Fig. 9 when the rotating speed is zero, the reaching rate is approximately 0:01 106 mol1 dm2 s1 . If the liquid is idealized solution the leaching rate should be zero when the zinc oxide specimen is stationary. The leaching rate is not zero because even rotating speed of the specimen is zero the natural convection occurs by the difference of concentrate of Leaching of Zinc Oxide in Acidic Solution chemicals between on the surface of the specimen and bulk solutions. These experimental data that the activation energy is rather low and the leaching rate depends on the rotating speed of zinc oxide disk indicate that the rate controlling step of zinc oxide leaching in hydrochloric and sulfuric acid is mass transfer through liquid boundary layer. Further investigation is necessary to determine the chemicals whose diffusion rate controls the leaching reaction rate. 4. Conclusions The leaching of zinc oxide disk in hydrochloric acid and sulfuric acid solutions was studied kinetically. The effect of pH value, temperature and rotating speed of zinc oxide disk on leaching rate were investigated. The followings are the results obtained from experimental data; (1) Leaching rate increases at lower pH value. (2) Leaching rate increases at higher leaching temperature. By the Arrhenius plot, the activation energy of the zinc oxide leaching by hydrochloric acid is 11.6 kJmol1 and 17.5 kJmol1 by sulfuric acid leaching. (3) Leaching rate increases in proportion to square root of 2493 rotating speed of zinc oxide disk. (4) The relatively lower activation energy and relationship between leaching rate and rotating speed of test specimen indicate that the reaction rate of zinc oxide leaching is controlled by mass transfer through the liquid boundary layer. REFERENCES 1) K. Matsumoto, S. Taniguchi and A. Kikuchi: J. Japan Inst. Metals 55 (1991) 853-859. 2) F. M. Doyles, N. Ranjan and E. Peters: Trans. Instn. Min. Metall. (Sect. C: Mineral Process. Extr. Metall.) 96 (1987), C69. 3) H. Gerischer and N. Sorg: Electrochemical Acta. 5 (1992) 827-835. 4) A. J. Monhenius and G. Katungu: Inst. Chem. Eng. Symp. Ser. 87 (1984) 635-42. 5) T. Yoshida: Shigen-to-Sozai 113 (1997) 967-971. 6) Y. Umetsu: Shigen-to-Sozai N5 (1995) 14-17. 7) H. Majima, Y. Awakura and T. Mishima: Metall. Trans. B 16B (1985) 23-30. 8) T. Hirato, H. Majima and Y. Awakura: Metall. Trans. B 18B (1987) 31-39. 9) T. Hirato, H. Hiai, Y. Awakura and H. Majima: Metall. Trans. B 20B (1989) 485-491.