Molecular Weight of Acetone
advertisement

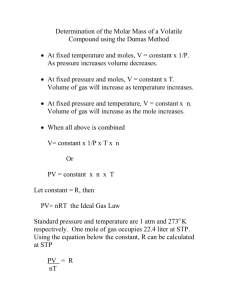
Molecular Weight of Acetone Name ______________________ Introduction: All gases take up the same volume at equal temperature and pressure. If we know how many moles of a gas are present and the weight of that gas, we can then know the molar mass of that gas. In a nutshell, we will be attempting to find the molar mass of acetone. To do this, we will place a few mL of acetone into a 250 – 500 mL flask. We will place an aluminum foil seal on the flask with a tiny pinhole. We will then gently heat the acetone to make it evaporate. The idea is that as gas effuses through the pinhole, the flask will fill with pure acetone. Procedure 1. 2. 3. 4. 5. 6. Place a few mL of acetone in a 250 – 500 mL flask. Cover flask with aluminum foil Make a pinhole with a tack or other device in aluminum foil Gently heat acetone and flask When all the acetone is evaporated, remove flask from heat and start to cool flask to 0 C Determine the mass of liquid (acetone) which reforms. Calculations 1. Determine the amount of moles of gas that would occupy your flask at approximately 40 C (approximated temperature of interior of flask). 2. Using a proportion, determine how what the mass of 1.0 moles of acetone would have. Further practice 1. A certain gas occupies a volume of 350 mL at a temperature of 0 C and 1 atm. If the gas has a mass of 1.2 g, what is the molar mass of the gas? 2. A certain gas occupies a volume of 1.0 L at a temperature of 0 C and 1 atm. If the gas has a mass of 2.0 g, what is the molar mass of the gas? 3. A certain gas occupies a volume of 500 mL at a temperature of 40 C and 1 atm. If the gas has a mass of 1.4 g, what is the molar mass of the gas?