Ex 3 Aspirin
advertisement

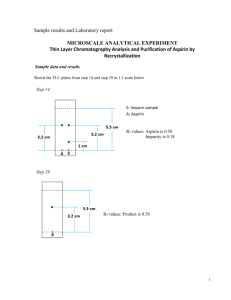
Doctrine of Signatures Auroleus Phillipus Theostratus Bombastus von Hohenheim "Paracelsus" Doctrine of Signatures - a principle that assigns healing properties to plants on the basis of the association between their physical characteristics and those of the disease or the affected part of the body. ‘Nature marks each growth… according to its curative benefit.’ Paracelsus noticed how the qualities of plants so often reflect their appearance – that the seeds of skullcap, for example, resemble small skulls and, it transpires, are effective at curing headache. Similarly, the hollow stalk of garlic resembles the windpipe and is used for throat and bronchial problems. By the same token, willow grows in damp places and will heal rheumatic conditions, caused by a build-up of fluid on the joints. Salicylic Acid and Derivatives 400 BC Hippocrates prescribed bark and leaves of the willow tree to reduce pain and fever. 1763 AD Revered Edward Stone - while suffering from various ‘agues’, was prompted to nibble a piece of bark from a willow tree and was struck by its extremely bitter taste. Knowing the bark of the Peruvian cinchona tree (quinine ) has a similarly bitter taste, he surmised that the willow might also have therapeutic properties by the "Doctrine of Signatures" - whereby the cause of a disease offers a clue to its treatment. H OH HO OH HO H H OH O OH H Salicin (analgesic f rom willo w bark) "As this tree delights in a moist or wet soil, where agues chiefly abound, the general maxim that many natural maladies carry their cures along with them or that their remedies lie not far from their causes was so very apposite to this particular case that I could not help applying it; and that this might be the intention of Providence here, I must own, had some little weight with me". "An Account of the Success of the Bark of the Willow in the Cure of Agues. In a Letter to the Right Honourable George Earl of Macclesfield, President of R. S. from the Rev. Mr. Edmund Stone, of Chipping-Norton in Oxfordshire" published in the Philosophical Transactions Volume 53 by the Royal Society of London 1763 Aspirin Salicin (willow bark) can be hydrolyzed/oxidized to glucose and salicylaldehyde (meadosweet plant, Spirea species). Salicylic acid was named spirsaure (Spirea + saure) by German discoverers but called salicylic acid by the English who traced its chemical history back to the willow tree. H2 C OH O C O HO O O H OH C O OH OH HO Salicylaldehyd e Salicy lic Acid OH O O OH Salicin C OH C C H3 Aspirin (Acety lsalicylic Acid) Dissociation of Carboxylic Acids Aspirin (acetylsalicylic acid) – member of compound called salicylates used in medicine for its analgesic (pain-relieving), anti-pyretic (fever-reducing) and antiinflammatory effects. O C O CH 3 C O benzoic acid H C CH 3 O O O C O Base H C O O O aspirin (acetylsalicylic acid) conjug ate base n eu tral fo rm This f orm exis ts in th e stomach ionic fo rm This f orm exis ts in th e intestines After ingestion, aspirin first travels into the stomach and then the intestines. In the acidic environment of the stomach, aspirin remains in its neutral form, but in the basic environment of the small intestine, aspirin is deprotonated to form its conjugate base, an ion. To be active, aspirin must cross a cell membrane, and to do so, it must be neutral, not ionic. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Two different cyclooxygenase isozymes (COX-1 and COX-2) are responsible for prostaglandin synthesis. COX-1 is involved with the usual production of prostaglandins and plays a role as a ‘housekeeping enzyme’ in maintaining the lining of the stomach and in endothelial cells contributing to the normal function of the cardiovascular system via release of prostacyclin (PGI2). In contrast, COX-2 is induced by specific stimuli and is thought to be involved in inflammation and mitogenesis responses. NSAIDs like aspirin and ibuprofen (Advil, Motrin, Nuprin) and naproxen (Aleve) inactivate both the COX-1 and COX-2 enzymes. This activity also results in an increase in gastric secretions, making an individual more susceptible to ulcer formation. O O C O2H CO2H CO 2H H3C O Aspirin Ibup ro fen Naprox en Aspirin A transesterification reaction that blocks prostaglandin synthesis is responsible for aspirin’s activity as an anti-inflammatory agent. Prostaglandins have several functions, one of which is inflammation. The enzyme prostaglandin synthase catalyzes the conversion of arachiodonic acid into PGH2 a precursor of prostaglandins and thromboxanes. Prostaglandins Arachiodonic acid Prostaglandin synthase PGH2 Thromboxanes Prostaglandin synthase is composed of two enzymes, one of which (cyclooxygenase) has a serine –CH2OH which reacts with aspirin to inactivates the enzyme. Prostaglandins cannot be synthesized and inflammation is suppressed. Thromboxanes stimulate platelet aggregation, and this is why low levels of aspirin is believed to reduce the incidence of strokes and heart attacks that result from blood clot formation (anticoagulant) - COO O C CH3 O acetylsalicylate aspirin H2 + HO C enzyme (active) transesterification COO O - OH salicylate H3C C + H2 O C acetylated enzyme (inactive) Acetylation Acetic anhydride forms acetate esters from alcohols and N-substituted acetamides from amines O CH3 C O O O C HO CH2CH3 + CH3 Acetic Anhydride (Ac2O) + CH3 + CH3 OCH2CH3 C C O O O O NH2 C Ethyl Acetate O OH CH3 C CH3 O C CH3 CH3 O H N O C C O CH3 Acetylation With reference to the structures of acetylsalicylic acid (aspirin) and acetaminophen (Tylenol) explain each statement: a) Acetaminophen tablets can be stored in the medicine cabinet for years, but aspirin slowly decompose over time; b) Children’s Tylenol can be sold as a liquid (acetaminophen dissolved in water), but aspirin cannot. OH Acetylsalicylic Acid (Aspirin) CO2H O CH3 C O O O C C CH3 O CO2H CH3 H N Acetic Anhydride NH2 HO HO C CH3 O Acetaminophen (Tylenol) Acetaminophen reduces pain and fever, but it is not anti-inflammatory, so it is ineffective in treating conditions like arthritis, which has a significant inflammatory component. In larger doses, acetaminophen causes liver damage, so dosage recommendations must be carefully followed. Preparation of Aspirin and IR Spectroscopy Scheme: O OH C + OH H3C O O C C O C CH 3 O H2SO 4 (cat) CH 3 C O O O H + H 3C C O salicylic acid acetic anhydride O-acetylsalicylic acid Aspirin acetic acid Acetic anhydride forms acetate esters from alcohols O CH 3 OH C O O O C CH 3 Acetic Anhy dride (Ac2O) + HO CH2C H3 CH 3 C OCH 2CH3 Ethyl Acetate Preparation of Aspirin and IR Spectroscopy • Infrared Spectroscopy – To determine the presence or absence of functional groups or bonds in a molecule – Covalent bonds behave as springs – Springs bend and stretch – Bending and stretching vibrations within covalent bonds cause them to absorb infrared radiation. • Typical IR spectrum • Plot of percent transmittance vs. frequency of vibration • Frequency is an energy given in wavenumbers (cm-1) where wavelength is expressed in cm. Preparation of Aspirin and IR Spectroscopy Common IR absorptions Regions Bond or Functional Groups Comments ~3350 cm-1 O-H stretch Broad, intense ~3200 – 2800 cm-1 O-H stretch from CO-OH dimer Single broad absorption which often swallows other peaks ~3150 – 3050 cm-1 Aromatic C-H stretch Usually several ~2950 – 2850 cm-1 Aliphatic C-H stretch Usually several ~1700 cm-1 C=O stretch One single strong peak Exact region depends on type of C=O ~1600 cm-1 and ~1500 cm-1 Aromatic C=C stretch Peak at ~1600 cm-1 usually split into a doublet ~600 – 850 cm-1 Aromatic C-H bending Usually several peaks, intense Preparation of Aspirin and IR Spectroscopy • • Procedure, in “Catalyst”, pg 146 - 147 Do not use water baths Purification • 1) Disperse sample in sodium bicarbonate O C O CH 3 O • • CH 3 O O C C O NaHCO 3 H C O O + O C CH 3 O H C O H O 2) Filter by vacuum – Waste disposal: DO NOT POUR FILTRATE DOWN THE DRAIN. This filtrate is what must be acidified in the next step. The book is wrong. 3) Ferric Chloride test – a test for phenols – A positive test is violet color with FeCl3 – A negative test is not more than a light violet color Preparation of Aspirin and IR Spectroscopy • Identification of the product O C CH3 carbo nyl of an ester O C O • • • O H arom atic C=C carbon yl of a carbox ylic acid H H O C H H aro matic C-H aliph atic (sp 3) C-H For preparing samples: ~ 1 mg of sample per 100 mg KBr In your reports, don’t characterize by melting point. Report procedure, no comparison to literature melting point.