Burning Magnesium Demonstration - C7Chemistry
advertisement
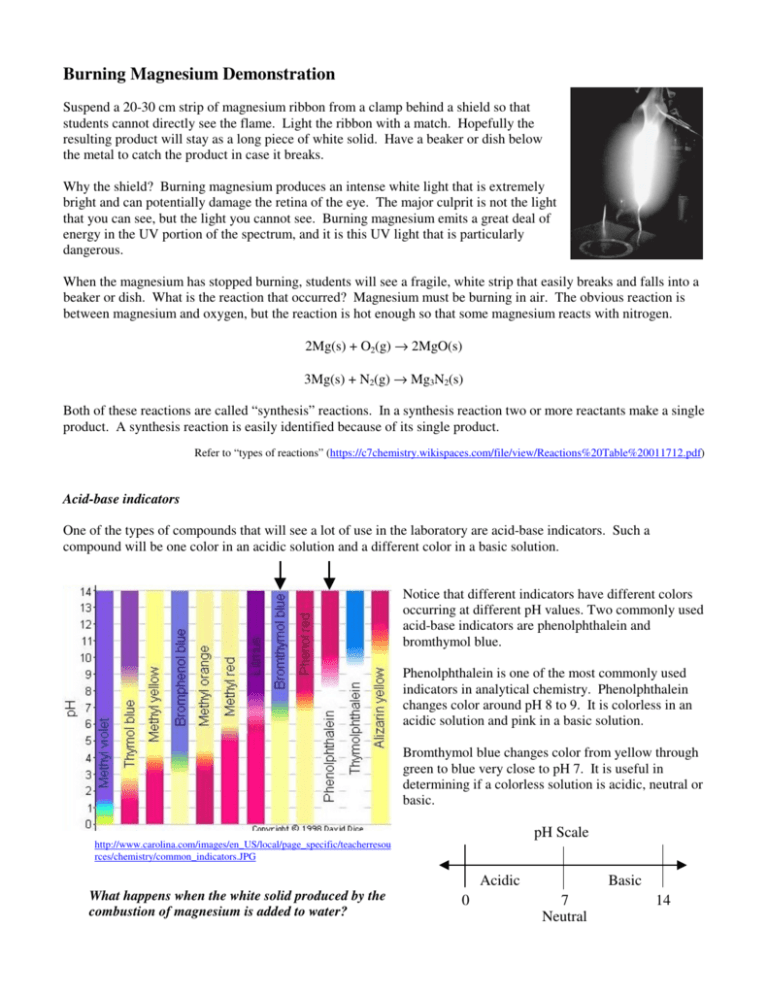
Burning Magnesium Demonstration Suspend a 20-30 cm strip of magnesium ribbon from a clamp behind a shield so that students cannot directly see the flame. Light the ribbon with a match. Hopefully the resulting product will stay as a long piece of white solid. Have a beaker or dish below the metal to catch the product in case it breaks. Why the shield? Burning magnesium produces an intense white light that is extremely bright and can potentially damage the retina of the eye. The major culprit is not the light that you can see, but the light you cannot see. Burning magnesium emits a great deal of energy in the UV portion of the spectrum, and it is this UV light that is particularly dangerous. When the magnesium has stopped burning, students will see a fragile, white strip that easily breaks and falls into a beaker or dish. What is the reaction that occurred? Magnesium must be burning in air. The obvious reaction is between magnesium and oxygen, but the reaction is hot enough so that some magnesium reacts with nitrogen. 2Mg(s) + O2(g) → 2MgO(s) 3Mg(s) + N2(g) → Mg3N2(s) Both of these reactions are called “synthesis” reactions. In a synthesis reaction two or more reactants make a single product. A synthesis reaction is easily identified because of its single product. Refer to “types of reactions” (https://c7chemistry.wikispaces.com/file/view/Reactions%20Table%20011712.pdf) Acid-base indicators One of the types of compounds that will see a lot of use in the laboratory are acid-base indicators. Such a compound will be one color in an acidic solution and a different color in a basic solution. Notice that different indicators have different colors occurring at different pH values. Two commonly used acid-base indicators are phenolphthalein and bromthymol blue. Phenolphthalein is one of the most commonly used indicators in analytical chemistry. Phenolphthalein changes color around pH 8 to 9. It is colorless in an acidic solution and pink in a basic solution. Bromthymol blue changes color from yellow through green to blue very close to pH 7. It is useful in determining if a colorless solution is acidic, neutral or basic. pH Scale http://www.carolina.com/images/en_US/local/page_specific/teacherresou rces/chemistry/common_indicators.JPG Acidic What happens when the white solid produced by the combustion of magnesium is added to water? 0 Basic 7 Neutral 14 The white solid is added to a solution of water and phenolphthalein indicator. The pink color of the phenolphthalein indicator shows that a reaction has occurred that makes the solution basic. The water is neutral and so the phenolphthalein is colorless. The magnesium oxide reacts with water to make an aqueous solution of magnesium hydroxide. The reaction is a single replacement reaction. MgO(s) + H2O(l) → Mg(OH)2(s) The resulting magnesium hydroxide is not very soluble in water, but what does dissolve in water dissociates into ions. We write the reaction as an equilibrium with a double arrow to indicate that only some of insoluble Mg(OH)2 molecules split up into ions. Mg(OH)2(s) Mg2+ + 2OHThe better equation for the reaction of MgO and water is the ionic equation shown above. The ionic equation represents the substances as they actually occur. The presence of hydroxide ions shifts the pH of the solution so that it is basic. The basic solution causes the phenolphthalein to be dark pink. The interesting reaction is the one between magnesium nitride and water. Magnesium nitride reacts with water to make magnesium hydroxide and ammonia gas. Sometimes students can detect the odor of ammonia when a few drops of water are placed on solid magnesium nitride. The magnesium hydroxide partially dissociates in water to form magnesium ions and hydroxide ions. The solution is basic. Mg3N2(s) + 6H2O(l) → 3Mg(OH)2(aq) + 2NH3(g) Mg(OH)2(s) Mg2+ + 2OHAmmonia, NH3, is somewhat soluble in water and reacts with water to make a basic solution. NH3(aq) + H2O NH4+ + OH- Important: Notice that the ionic equations are the best representations of the substances in aqueous solution. Some questions about the reactions that follow the “burning of magnesium.” Answer the following on notebook paper. Make your answers as complete as possible. 1. Why should you not look directly at burning magnesium? 2. How is UV light different from visible light? 3. Approximately how hot can burning magnesium get? 4. Why is water never used on burning magnesium? 5. An acid-base indicator changes color with different pH values. Answer the following with “acidic”, “basic” or “neutral”. a. What are solutions with pH values below 7? b. What are solutions with pH values of 7? c. What are solutions with pH values above 7? d. Bromthymol blue is blue in what kind of solution? e. Bromthymol blue is green in what kind of solution? f. Bromthymol blue is yellow in what kind of solution? g. Phenolphthalein is colorless in what kinds of solutions? h. Phenolphthalein is pink in what kind of solution? i. What is a solution of magnesium hydroxide in water? j. What is a solution of ammonia in water? 6. Write the molecular equation for the reaction of magnesium burning in oxygen. 7. Write the molecular equation for the reaction of magnesium and nitrogen. 8. What about the two preceding reactions indicates that they are both synthesis reactions? 9. Write the molecular equation for the reaction of magnesium oxide and water. 10. What type of reaction is the one between magnesium oxide and water? 11. Write the ionic equation for the dissociation of magnesium hydroxide in water. 12. Write the molecular equation for the reaction of magnesium nitride and water. 13 Write the ionic equation for the reaction of ammonia and water. 14. What information does the double arrow () indicate that is different from the usual single arrow (→)? 15. Based on the chemical equations you have just written, explain why the phenolphthalein turns pink.






