How to Identify things on a periodic table
advertisement
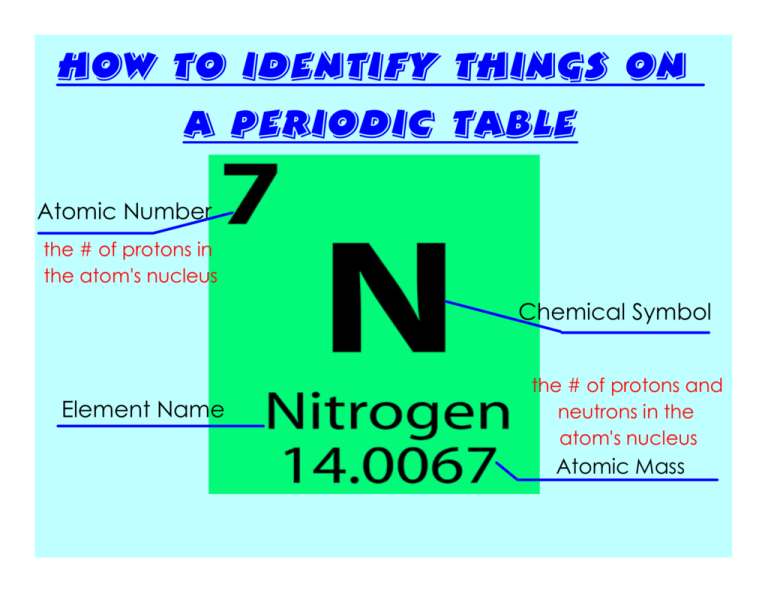
How to Identify things on a periodic table Atomic Number the # of protons in the atom's nucleus Chemical Symbol Element Name the # of protons and neutrons in the atom's nucleus Atomic Mass How to Read the Periodic Table: -Broken into 18 groups & 7 periods. *REMEMBER: groups- vertical periods- horizontal -Groups are related by chemical and physical properties, whereas periods are not. What are these groups known as? Group 1- Alkali Metals Group 2- Alkaline Metals Groups 3-12: Transition Metals Group 17- Halogens Group 18- Noble Gases Key Terms from Chapter 1: Let's Try a Few Together! :) 1. What types of charges do protons, neutrons, and electrons have? PROTONS- Positive NEUTRONS- Neutral (no charge) ELECTRONS- Negative 2. Where can these particles be found? PROTONS- Inside the nucleus NEUTRONS- Inside the nucleus ELECTRONS- Orbit outside the nucleus Key Terms from Chapter 1: Let's Try a Few Together! :) 3. Who was the British scientist that created the idea of the atom? John Dalton 4. Who was the Russian scientist that came up with the idea of the periodic table and how to arrange it? Dmitri Mendeleev 5. How is the periodic table organized? It is organized by atomic number and also by certain properties of the elements. Key Terms from Chapter 1: Let's Try a Few Together! :) 6. What are some characteristics of metals? Most are shiny, easily shaped, and are good conductors of heat and electricity. 7. What are some characteristics of non-metals? Opposite to metals--NOT good conductors of electricity or heat. *Most have the ability to gain electrons easily* 8. What are some characteristics of metalloids? Contain qualities of both metals and non-metals. Silicon, for example, possesses a metallic luster, yet it is an inefficient conductor and is brittle. Key Terms from Chapter 1: Let's Try a Few Together! :) 9. How can positive ion form? When an atom LOSES one or more electrons. 10. How can a negative ion form? When an atom GAINS one or more electrons. 11. What is an isotope? When the atoms of the same element have a different number of neutrons. Find your key! This will help you determine the element's details. :) NOTE: not every periodic table is the same! Make sure you identify the key. TOMORROW YOU WILL NOT BE GIVEN THE GROUPS/PERIODS ARROWS! What group/period is Zinc found in? Group 12; Period 4 What element has 56 protons? Barium Please tell me the chemical symbol of the element that has 81 protons. Tl- Thallium Find the element at Group 12, Period 6. What is its state of matter at room temperature? Liquid Tell me the group and period... I am found in the Halogen group and am a liquid at room temperature . Group 17, Period 4 (Bromine) What is the atomic mass of the element found at Group 2, Period 2? 9.012 Find the element at Group 5, Period 7 and determine the # of neutrons in the atom's nucleus 157 Neutrons Atomic mass - Atomic Number 262 - 105 In an average atom of Chromium, there are 24 protons. How many electrons would there be? 24 Electrons How to Read the Periodic Table by Group/ Period (Open Textbooks to p. 20): Let's Try a Few Together! :) 1. Find the element at group 14, period 2. What is the atomic number of this element? Carbon- 6 2. Find the element at group 17, period 5. What is the chemical symbol of this element? Iodine- I 3. Find Lithium on the periodic table. What state of matter is it at room temperature? Solid 4. Find the element in Group 3 Period 7. Is it a metal, non metal, or metalloid? Metal 5. Find the element at group 8, period 4. What is its atomic mass? Iron- 55.845