Chemistry A Midterm Review (Key)
advertisement
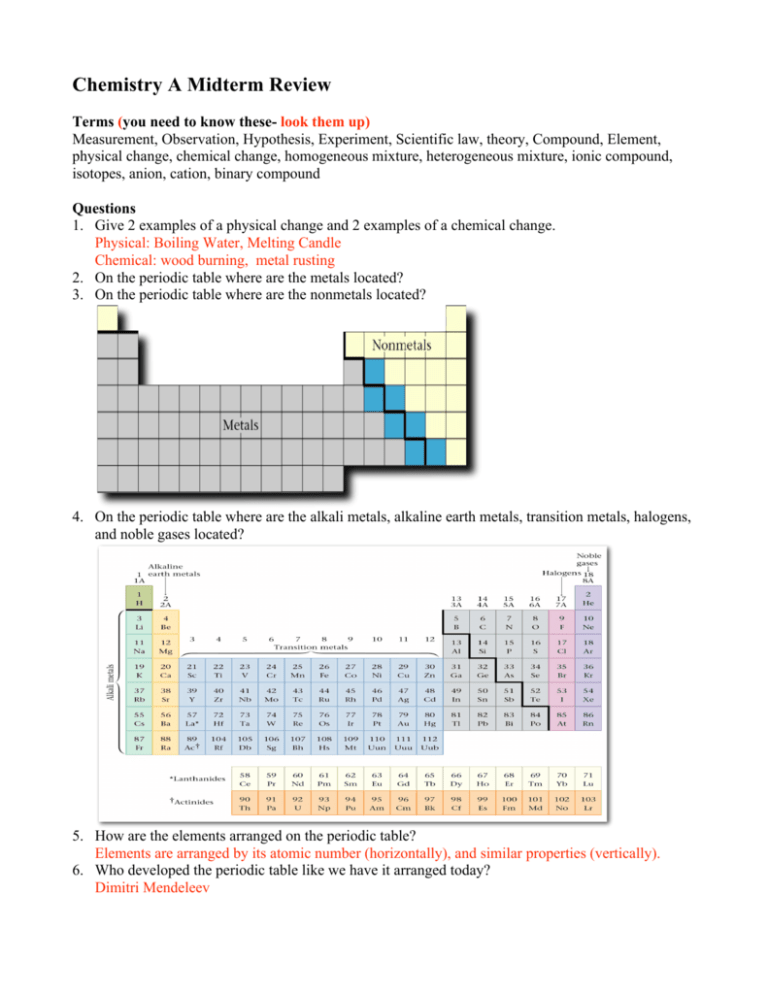
Chemistry A Midterm Review Terms (you need to know these- look them up) Measurement, Observation, Hypothesis, Experiment, Scientific law, theory, Compound, Element, physical change, chemical change, homogeneous mixture, heterogeneous mixture, ionic compound, isotopes, anion, cation, binary compound Questions 1. Give 2 examples of a physical change and 2 examples of a chemical change. Physical: Boiling Water, Melting Candle Chemical: wood burning, metal rusting 2. On the periodic table where are the metals located? 3. On the periodic table where are the nonmetals located? 4. On the periodic table where are the alkali metals, alkaline earth metals, transition metals, halogens, and noble gases located? 5. How are the elements arranged on the periodic table? Elements are arranged by its atomic number (horizontally), and similar properties (vertically). 6. Who developed the periodic table like we have it arranged today? Dimitri Mendeleev 7. What is a family on the periodic table? A vertical column on the periodic table. 8. Write the name and atomic number for each of the following elements: a. He - helium 2 c. Se- selenium 34 e. P- phosphorus 15 b. B – boron 5 d. Ba- barium 56 f. Sr- strontium 38 9. For each of the following pairs of ions, write the formula for the ionic compound. a. Rb+ S2- Rb2S b. Fe2+ Cl - FeCl2 c. Al3+ O2- Al2O3 10. How many oxygen atoms are there in the following compounds? a. CO 1 b. NH4NO3 -3 c. Al(NO3)3 9 d. N2O3 3 11. What are the three fundamental particles that compose all atoms? Indicate the electrical charge and where is each type of particle found in the atom? Particle Charge Location Proton + nucleus Neutron 0 nucleus Electron orbits nucleus 12. The atomic number tells us how many _protons__ are in the nucleus of an atom. 13. The mass number of an isotope is the sum of _protons and neutrons___. 14. Give the common ions that the following elements form. 15.a. K - K+ b. O- O2c. Ca- Ca2+ d. Cl- Cl15. In ionic compounds, is the metal or nonmetal named first? Metal 16. What are the standard SI units? 17. Know the metric prefixes. 18. For each of the following, make the indicated conversion. a. 5.993 × 10-4 to ordinary decimal notation 0.0005993 4 b. 4.321 × 10 to ordinary decimal notation 43210 c. 5240000000 to standard scientific notation 5.24 x 109 d. 0.0000009814 to standard scientific notation 9.814 x10-7 19. How many significant figures are there in the following numbers? a. 0.0034570 5 b. 1234000 4 c. 0.0560789 6 d. 123056.00 8 e. 1.25 x 1034 3 20. Evaluate each of the following mathematical expressions, being sure to express the answer to the correct number of significant figures. a. 10.20 + 4.1 + 26.0001 + 2.4 42.7 b. 1.091 – 0.991 –1.2 -1.1 c. (67.21) (1.003) (2.4) 160 d. (1.674) / (1.1236) 1.490 21. Make the indicated temperature conversions. a. 541 K to Celsius degrees 427 K b. 221 °C to kelvins 498 oC c. – 50.1°C to Fahrenheit degrees -58.2 oF d. – 30.7 °F to Celsius degrees -34.8 oC 22. Given the following mass, volume, and density information, calculate the missing quantity. a. mass = 121.4 g; volume = 42.4 cm3; density = ? g/cm3 2.66 g/cm3 b. mass = ? g; volume = 124.1 mL; density = 0.821 g/mL 102 g c. mass = 142.4 g; volume = ? mL; density = 0.915 g/mL 156 ml 23. For each of the following, make the indicated conversion a. 88.5 cm to millimeters (100 cm=1m) (1000mm= 1m) 885 mm b. 8.25 m to inches (100cm= 1m) (2.54cm= 1in) 325 in c. 4.25 kg to pounds (1kg= 2.205lbs) 9.37 lbs d. 4.21 in. to centimeters (2.54cm= 1in) 10.7 cm 24. Name each of the following compounds. NaCl sodium chloride MgBr2 magnesium bromide SnO2 tin (IV) oxide B2O3 diboron trioxide HClO4 perchloric acid AlI3 aluminum iodide CO carbon monoxide NH4NO3 ammonium nitrate FeCl3 iron (III) chloride Al(NO3)3 aluminum nitrate N2 O3 dinitrogen trioxide BaCl2 barium chloride Ni3(PO4)2 nickel (II) phosphate CuO copper (II) oxide