Menu
Lesson
Print
Name ______________________________________ Date ____________ Class _______________________
Modern Chemistry • CHAPTER 12
HOMEWORK 12-3
(pp. 273–375)
VOCABULARY
Fill in the blank in each sentence.
1. Equilibrium is ____________________________________________________________________
_________________________________________________________________________________.
2. A phase is any part of a system that has ________________________________________________.
3. The process in which a gas changes to a liquid is _________________________________________.
4. __________________, __________________, and __________________ are all examples of changes
of state.
5. Write Le Châtelier’s principle. ______________________________________________________
_________________________________________________________________________________.
SKILL BUILDER
Label the process for each of the changes of state. Then give two examples of that change of state.
Your examples should be different from those listed in Table 12-2.
Change of State
Process
Examples
Solid Æ gas
Gas Æ liquid
Liquid Æ solid
Gas Æ solid
Solid Æ liquid
Liquid Æ gas
STANDARDIZED TEST PREP
Circle the letter of the best answer.
1. The rate at which molecules pass from the vapor phrase to the liquid phase depends on the
a. temperature of the molecules.
b. boiling point of the liquid.
c. volume and kinetic energy of the molecules.
d. concentration of molecules in the vapor phase.
2. Which statement is not true?
a. Matter cannot enter or leave a closed system.
b. Matter can escape from an open system.
c. Energy cannot enter a closed system.
d. Energy can enter an open system.
Modern Chemistry
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Menu
Lesson
Print
Name ______________________________________ Date ____________ Class _______________________
Modern Chemistry • CHAPTER 12
HOMEWORK 12-4
(pp. 376–379)
VOCABULARY
Define.
1. volatile liquid ______________________________________________________________________
__________________________________________________________________________________
2. equilibrium vapor pressure ____________________________________________________________
__________________________________________________________________________________
3. molar heat of vaporization ____________________________________________________________
__________________________________________________________________________________
4. boiling ____________________________________________________________________________
__________________________________________________________________________________
5. boiling point _______________________________________________________________________
__________________________________________________________________________________
GRAPHIC ORGANIZER
On a separate sheet of paper, draw a diagram to show the various changes that occur as a liquid is
heated to its boiling point. Be sure to include changes in temperature, vapor pressure, and so on.
Explain what happens to the process if the pressure above the liquid is increased.
____________________________________________________________________________________
____________________________________________________________________________________
STANDARDIZED TEST PREP
Circle the letter of the best answer.
1. Which happens to a liquid as its temperature increases?
a. Its volume increases.
b. The liquid is sublimated.
c. The equilibrium vapor pressure increases.
d. The kinetic energy of the liquid decreases.
2. Nonvolatile liquids evaporate slowly because
a. they are more dense than volatile liquids.
b. they are covalently bonded crystalline structures.
c. their boiling points are relatively high.
d. the attractive forces between the particles are strong.
Modern Chemistry
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Menu
Lesson
Print
Name ______________________________________ Date ____________ Class _______________________
Modern Chemistry • CHAPTER 12
HOMEWORK 12-5
(pp. 379–381)
VOCABULARY
Complete each sentence.
1. The normal freezing point of a substance is _____________________________________________
_________________________________________________________________________________.
2. The amount of heat required to melt one mole of solid at its melting point is its ________________
_________________________________________________________________________________.
3. Sublimation is ____________________________________________________________________.
4. The reverse process of sublimation is called _____________________________________________.
SKILL BUILDER
For each of these processes, write an equilibrium equation that includes a heat term. The first is
done for you.
Condensation
gas
liquid + heat
Melting
Freezing
Deposition
Sublimation
Vaporization
STANDARDIZED TEST PREP
Circle the letter of the best answer.
1. Which statement about freezing point is not true?
a. The normal freezing point value depends upon a pressure of 101.3 kPa.
b. At the freezing point, the liquid has more kinetic energy than the solid.
c. The energy loss during freezing is a loss of potential energy that was present in the liquid.
d. The particle order at the freezing point is significantly increased.
2. Water and ice remain at 0°C as long as both water and ice are present. Adding heat causes a higher
proportion of water than ice. This phenomenon is explained by
a. the process of sublimation.
b. Charles’s law explaining the volume-temperature relationship.
c. the loss of energy as heat by the liquid.
d. Le Châtelier’s principle.
Modern Chemistry
Copyright © by Holt, Rinehart and Winston. All rights reserved.
Menu
Lesson
Print
Name
Date
Class
CHAPTER 12 REVIEW
Liquids and Solids
SECTION 12-3
SHORT ANSWER
Answer the following questions in the space provided.
1. Consider the following system at equilibrium:
→ products
reactants ←
A change in conditions causes the reverse reaction to be favored.
a. What happens to the concentration of the reactants?
b. What happens to the concentration of the products?
2. The molar heat of vaporization of methane, CH4, is 8.19 kJ/mol; for water, it is 40.79 kJ/mol.
a. If 2.0 1023 molecules of liquid CH4 are made to boil, how much
heat must be supplied? Show all your work.
b. Based on the molar heat of vaporization data, which is more
volatile, CH4 or H2O?
c. Which molecule is more polar, CH4 or H2O?
3. A general equilibrium equation for boiling is:
→ vapor
liquid heat energy ←
Is the forward or reverse reaction favored in each of the following cases?
a. The temperature of the system is increased.
b. More molecules of the vapor are added to the system.
c. The pressure on the system is increased.
4. Consider water boiling in an open pot on a stove.
a. Can this system reach equilibrium? Why or why not?
MODERN CHEMISTRY
HRW material copyrighted under notice appearing earlier in this work.
SECTION 12-3 REVIEW
103
Lesson
Menu
Print
Name
Date
Class
SECTION 12-3 continued
b. Explain the difference between an open system and a closed system.
5. Methanol has a normal boiling point of 65°C. It is a liquid at conditions of 1 atm and 25°C. A small
beaker filled with methanol is placed under a bell jar, and the air is then pumped out. It is observed
that under a vacuum the methanol boils readily at 25°C.
Use the kinetic-molecular theory and the concept of equilibrium vapor pressure to account for the
lowered boiling point of methanol under a vacuum.
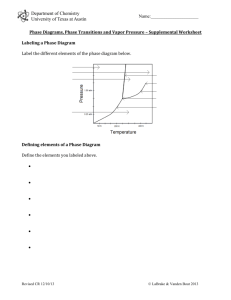
6. Refer to the phase diagram for water in Figure 12-14 on page 381 of the text to answer the
following questions:
a. Which point represents the conditions under which all three phases
can coexist?
b. Which point represents a temperature above which only the solid
phase exists?
c. Based on the diagram, as the pressure on the water system is
increased, the melting point of ice
(increases, decreases, or
stays the same).
104
SECTION 12-3 REVIEW
MODERN CHEMISTRY
HRW material copyrighted under notice appearing earlier in this work.
Menu
Lesson
Print
Name ______________________________________ Date ____________ Class _______________________
Modern Chemistry • CHAPTER 17
HOMEWORK 17-1
(pp. 511–514)
VOCABULARY
Define.
1. temperature ________________________________________________________________________
__________________________________________________________________________________
2. heat ______________________________________________________________________________
__________________________________________________________________________________
3. specific heat _______________________________________________________________________
__________________________________________________________________________________
4. calorimeter ________________________________________________________________________
__________________________________________________________________________________
5. thermochemistry ____________________________________________________________________
__________________________________________________________________________________
SKILL BUILDER
1. Explain the difference between temperature and heat. ______________________________________
__________________________________________________________________________________
Solve. Show your work on a separate sheet of paper.
2. The specific heat of aluminum is 0.897 J/(g·K). If a 22.6 g sample of aluminum is heated from 183 K
to 244 K, then how much heat will the aluminum absorb?
3. 1.3 kg of a substance is heated from 269 K to 325 K and is found to have absorbed 45 J of heat.
What is the specific heat of this substance?
4. The specific heat of mercury is 0.140 J/(g·K). If 450 kJ of energy is added to 43 g of mercury at
315 K, what will the final temperature of the mercury be?
STANDARDIZED TEST PREP
Circle the letter of the best answer.
1. Specific heat is usually measured
a. at constant temperature.
b. for a particular volume of a substance.
c. in degrees Celsius or kelvins.
d. at constant pressure.
2. A 2.5 kg sample of a substance was heated from 113°C to 289°C. The substance absorbed 0.45 kJ
of heat. What is the specific heat of this substance?
a. 1.02 x 10–3 J/g·K
b. 1.98 x 105 J/g·K
c. 1.02 x 10–6 J/g·K
d. 6.4 x 10–3 J/g·K
Modern Chemistry
Copyright © by Holt, Rinehart and Winston. All rights reserved.