Experiments - University of Illinois Archives
advertisement

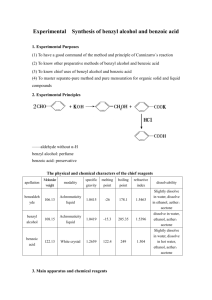
Schedule of Experiments Experiment Schedule Chemistry 234 - Spring 2001 Text: Experimental Organic Chemistry by J. C. Gilber and S. F. Martin. Publisher: Saunders, 1997, 2nd Edition PART 1 - Introduction to Laboratory Techniques (55 points) Jan 22 - 26 Jan 29- Feb 2 Feb 5- Feb 9 Lab Periods 1 1 1 Feb 12 - 16 1 Date Experiment Reading Credit Check In, Melting Points Recrystallization Fractional Distillation p 35-37, 105-109,syllabus p 87-105, syllabus p 113-134, syllabus p 68-71, p135-146, syllabus 10 10 15 Extraction 20 Part II - Synthesis (130 points) Feb 19- 23 1 Feb 26 - March 2 9 March 19- 23 1 2-chloro-2-methylpropane p 377-381, p 387-391, syllabus 20 methyl benzoate syllabus 40 methyl m-nitrobenzoate syllabus 25 March 26-30 1 Cyclohexene syllabus 20 April 2- 6 1 anisalacetophenone p 497-500, syllabus 25 Part III - Identification of Organic Compounds (45 points) April 9- 20 April 23 - 27 2 Identification of an aldehyde, amine, alcohol or ketone p 619-622, 630-632, 635-651, 661-665, 672-678, sylabus Make-up and checkout Hour Exams: 7:00 p.m. March 1 and April 25, 2001 file:///D|/Chem234/Syllabus/Schedule[1].htm [1/13/2001 10:59:05 AM] 45 General Information GENERAL INFORMATION Course Objectives Chem 234 provides you with an opportunity to learn about the properties, syntheses, separation, purification, and identification of organic compounds. The course consists of lecture sessions and laboratory sessions. In lecture sessions you learn the theory and detailed background behind certain organic experimental techniques. In laboratory sessions you conduct experiments designed to introduce you to a variety of these experimental techniques. Chem 234 is also designed to provide you with the opportunity to learn how to keep the records of scientific research in a laboratory notebook, a skill which is required in many scientific fields. You are, of course, expected to perform the assigned experiments in the laboratory, but you are also expected to understand the principles behind these experiments. Near the end of the course, you will be expected to carry out the chemical syntheses of organic compounds and determine the structures of unknowns. Experiments The techniques and background material for each experiment will be presented in the weekly lecture session. You must read the appropriate text and syllabus material before coming to each lecture You will perform the experiments only in your assigned laboratory period. We expect that you will come to each laboratory session well-prepared and ready to use your laboratory time efficiently. There are times when you may need to work on two different experiments in the same laboratory session, so you must plan ahead. If you miss a lab period for a valid reason (medical or a pre-approved absence), you may be able to do the experiments at another time (preferably that same week). However it is your responsibility to schedule these make-ups with your TA and with the Storekeeper. Unexcused absences cannot be made up and will be scored zero. Each new technique will be described to you in detail before you will have to use it, but thereafter you will be expected to be able to use that technique without any further detailed directions. To do this, you must acquire not only good manipulative skills in the laboratory but also a good sense about what a given procedure accomplishes and when it should be used. Occasionally, you may encounter difficulties with an experiment. If you are responsible for this difficulty and require additional laboratory time and/or starting material, a penalty of 10% of the grade for that experiment will be assessed. If, however, it is not your fault that you need extra time and/or material, no penalty will be assessed. file:///D|/Chem234/Syllabus/general.htm (1 of 3) [1/13/2001 10:38:38 AM] General Information Lab Quiz There is a on-line quiz associated with each experiment in WebCT. To receive credit for the quiz it must be completed before the scheduled starting time of your laboratory section. ChemNet Lessons ChemNet lessons are available to aid in your understanding of the experiments and to make your laboratory work easier. Although you can work at these lessons any time you wish, it is most helpful if you do the appropriate lessons shortly before going to the laboratory. You will earn 2 points toward your grade for each organic laboratory ChernNet lesson you complete. In Chem 234 the ChemNet lessons are on the Internet. You access them through WebCT. To log onto ChemNet select the ChemNet icon in WebCT. Next you have to type your NetID and choose a secret password that you must remember. The password may be the same as for your e-mail. You will be asked for this password each time you log onto ChemNet. The graded ChemNet lessons for Chem 234 are listed below (in the order you will want to complete them): Melting Points Phase Diagrams Mixed Melting Points Recrystallization Boiling Points Mole Fractions B.p. of Mixtures Distil Experiment Fractional Distillation Distillation Columns Extractions Extraction Experiment t-Butyl Chloride Chemical Tests Try Known Compound Qual Unknown Getting Help: There will be time at the end of each laboratory session to get help from your teaching assistant. In addition, TAs are available in the Chemistry Learning Center, 212 Chemistry Annex, Monday Thursday afternoons to answer questions. Missed Labs: For medical or true emergency reasons we will attempt to accommodate students who cannot carry out their experiments at the assigned times by scheduling them to do these experiments at other times. However, to receive credit for such experiments, the student must inform their own TA of the rescheduling and reschedule the experiment with the storeroom manager (469 Noyes Lab) at least 2 days file:///D|/Chem234/Syllabus/general.htm (2 of 3) [1/13/2001 10:38:38 AM] General Information in advance of the make-up date. Such rescheduling will only be provided for significant medical reasons or emergency situations. More than one rescheduled lab per semester will require the written approval of the instructor in charge of the course. Students will receive a grade of zero for labs not made-up and more than one zero on a lab will almost certainly result in failure of the course. file:///D|/Chem234/Syllabus/general.htm (3 of 3) [1/13/2001 10:38:38 AM] Grading Grading The grading in Chem 234 is based upon the results of the ten experiments, the two one-hour written exams, 10 on-line quizzes, and the 16 ChemNet lessons. The semester of Chem 234 is divided into three parts. Part 1, Introduction to Techniques, emphasizes theory and principles. Most of the points in Part I are earned on a 50 minute written exam covering these topics. Part II, Synthesis, emphasizes laboratory work. Most of the points in Part II are earned from the results (yields, purity, calculations) of the three experiments. Part III, Identification of Organic Compounds, also emphasizes laboratory work, with most of the points being based upon your ability to identify and support your identification of an unknown organic compounds. Near the end of the semester, the second (and last) 50 minute written exam will cover the theory and practice of the laboratory experiments in Parts 11 and 111, including the applications of spectroscopy. The points assoicated with these activities are: Activity Laboratory Work 10 for a total of 230 points On-line Quizzes 10@10 points each 50 Minute Exams 2 @ 100 pts each ChemNet Lessons 16 @ 2pts each Total Points 230 100 200 36 566 The grade distribution for Chem 234 will be approximately 15% A, 30% B, 50% C, and 5% D plus E. Therefore, an "average" student will receive the "average" grade of C in this course. You must complete every experiment in order to receive at least a grade of D. Any requests for regrading of laboratory reports or quizzes must be submitted in writing within one week of the day it is handed out in class. No requests will be accepted after this time period. All laboratory reports to be regraded should be submitted to your TA. If you cheat on any lab (i.e., report false data for the melting points or gram yields of the samples you turn in), you will automatically receive a grade of zero for that experiment andlor an F grade for this course. Your TA will check the melting point and gram yield of your samples. On-line quizzes must be completed before the start of your scheduled laboratory period. file:///D|/Chem234/Syllabus/Grading.htm [1/13/2001 10:40:27 AM] Laboratory Notebooks and Reports Laboratory Notebooks and Reports Your laboratory notebook is the complete and permanent record of your activities in the laboratory. Your notebook should be patterned after the form discussed in Chapter I of the textbook. All data, observations, etc., should be entered directly into the laboratory notebook during the laboratory session. As part of your pre-laboratory preparation, you should enter the quantities and physical properties of all the compounds and solvents to be used for an upcoming experiment. Your notebook will not be turned in each week, but it will be checked during the semester by your TA. Your laboratory report is different from your laboratory notebook. Your report is a complete and readable summary of the experiment you have just performed. The report is written on a sheet of paper separate from your laboratory notebook. Each laboratory report is due one lab period after you finish the particular experiment. Laboratory reports should be turned in at the beginning of the laboratory session. A late laboratory report incurs a penalty of 10% of the grade for that experiment. No report will be accepted more than two lab periods after it is due unless extenuating circumstances prevail. Since your laboratory reports will be graded, you should write them legibly, clearly, and in good English. The laboratory report should state a reference to the procedure followed and list any changes that were made from the stated procedure. DO NOT copy the entire procedure into your report, as it is not expected and is a waste of time. DO include the following items: (1) Balanced equations for the chemical reactions, (2) Summaries of data and yields, including the appropriate calculations, (3) Graphs or tables, if appropriate, (4) A discussion of errors and their possible effects upon the results of the experiment, (5) Answers to all assigned exercises (see below), (6) Anything else assigned, and (7) Anything you feel is pertinent to a good summary of the experiment. file:///D|/Chem234/Syllabus/Notebooks.htm [1/13/2001 10:41:12 AM] LABORATORY SAFETY LABORATORY SAFETY Read Chapter 1, especially pp. 15-21, of the textbook before coming to your first laboratory session. You will fill out the Safety-device Location Worksheet during your first laboratory session. The importance of safety precautions in a Chemistry laboratory cannot be overemphasized. The following safety rules should be followed. You must use common sense when working with chemicals and laboratory apparatus. As safety is an integral part of this course, your TA will be grading you for compliance with these safety rules. Laboratory Safety Rules 1. You must NOT be in the laboratory without a TA present. The laboratory sessions are four hours in length. You will not be allowed in lab after your lab session has ended. 2. Safety goggles for eye protection must be worn in the laboratory at all times. Prescription eyeglasses (even with safety lenses) do not provide adequate eye protection, especially from the sides. Therefore, you will be removed from the laboratory if you are found without safety goggles covering your eyes. It is very strongly recommended that you NOT wear contact lenses of any kind in the laboratory. You should read the safety information on contact lenses and laboratory accidents posted outside the lab. Please check with your TA if you absolutely need to wear contact lenses. If you get anything in your eyes, wash your eyes thoroughly with plenty of water and notify your TA immediately. 3. Shoes MUST be worn in the laboratory at all times. Sandals or perforated shoes are not permitted, as broken glass and spilled chemicals are constant hazards. 4. Shorts and short skirts (above the ankle) will NOT be allowed. 5. You MUST note the location of the fire extinguishers, safety showers, eye washes, and first-aid kits in the laboratory, so that you will know where to obtain these items if they are needed. You will fill out the Safety-device Location Worksheet during your first laboratory session. 6. Many of the chemicals used in the laboratory experiments will be new to you. You should become acquainted with the properties of every new chemical you use. The Merck Index is a good source for finding the properties and toxicities of many organic compounds. All chemicals should be treated as though they are toxic. Compounds can enter the body by being absorbed through the skin, or by being inhaled or ingested. Therefore, (a) Keep vessels covered. Put the caps back on the solvent bottles immediately. Never evaporate solvents other than water into the atmosphere. Wipe up any spills immediately. In order to check for an odor, hold the sample about a foot away from your face and gently fan the vapors towards your nose. Do not put anything in your mouth. (b) You should use the gloves that are available. Keep your bench top clean! DO NOT rub your eyes or your face without first washing your hands. If something does get into your eyes, remember to wash your eyes with plenty of water and notify your TA. You should protect your clothing by wearing a laboratory file:///D|/Chem234/Syllabus/Safty.htm (1 of 7) [1/13/2001 10:41:59 AM] LABORATORY SAFETY apron. Always wash your hands thoroughly before leaving the laboratory session. If you have any cuts or scrapes, cover them with band-aids, etc., before coming to the laboratory. (c) To dilute acids, carefully and slowly add the concentrated acid to the water, never the other way around. This avoids dangerous splattering. "Do like you oughta, add acid to water". 7. Never heat a closed system! Always use boiling chips when heating any liquid, even water. When heating a test tube, never point it at yourself or at anyone else. Never heat flammable solvents (i.e., anything other than water) in an open container with a Bunsen burner. When you want to use a Bunsen burner, make sure that none of your neighbors is using a flammable solvent. Light the match first, then turn on the gas while holding the match close to the top of the burner. Long hair must be tied back. Do not wear garments with floppy sleeves or loose wrist cuffs. Turn off the Bunsen burner immediately when you are finished with it. 8. Do not use cracked or chipped glassware. Examine your glassware for "star" cracks. Broken glassware should be replaced immediately with new glassware from the storeroom. Do not handle broken glass with your hands. Sweep it up, or use a wad of toweling to grasp the pieces. 9. Only aqueous solutions (e.g., aqueous acids and bases) may be discarded down the drain. Flush them down with plenty of water. Other waste materials, both liquid and solid, should be disposed of into the labeled waste containers located in the hoods. Broken thermometers should be put into jars located in the hoods. Ask your TA to assist you in cleaning up any spilled mercury. 10. In the case of an accident (cuts, burns, reaction to a chemical, etc.), inform your TA immediately. A limited degree of first-aid is available in the storeroom, 469 Noyes Lab. If you are seriously injured, you will be taken to the medical center. Report all accidents immediately. Your health is more important than your grade in Chem 234! 11. Never pour chemicals directly from the storage containers directly into your reaction vessel. 12. Smoking, eating, and drinking are not permitted in the laboratory. 13. No pets are allowed in the laboratory. 14. No "horseplay" is allowed in the laboratory. 15. No radios are allowed in the laboratory. A good perception of your surroundings is very important in a chemical laboratory. This state of mind requires your full attention. Take care of yourself and your neighbors. Immediately warn your neighbor if you see him/her doing something dangerous. It is natural for you to feel somewhat confused at times. Do not be shy to ask your TA or the storeroom manager for guidance with the use of the laboratory equipment or for advice on safety matters. Please respect the fact that other students must use the common laboratory equipment, such as the balances, melting point apparati, hoods, etc. Take care of this equipment, and clean up your messes immediately. 111. Safety and Emergency Equipment In 467 Noyes Lab The Chem 234 laboratory sessions will be held in 467 Noyes Laboratory. This lab contains the following file:///D|/Chem234/Syllabus/Safty.htm (2 of 7) [1/13/2001 10:41:59 AM] LABORATORY SAFETY safety and emergency equipment: Fire Blanket Fire Extinguisher Overhead Shower file:///D|/Chem234/Syllabus/Safty.htm (3 of 7) [1/13/2001 10:41:59 AM] LABORATORY SAFETY Eye-Wash Faucets Fire Alarm First Aid Kit file:///D|/Chem234/Syllabus/Safty.htm (4 of 7) [1/13/2001 10:41:59 AM] LABORATORY SAFETY Before you begin laboratory work in this course, you must complete the Safety device Location Worksheet. Locate each of the items listed above and mark their locations on the Worksheet by using the symbols shown above. Indicate where your own area is located, and then indicate where the six nearest Eye-Wash Faucets are in relation to your work area. file:///D|/Chem234/Syllabus/Safty.htm (5 of 7) [1/13/2001 10:41:59 AM] LABORATORY SAFETY You must wear appropriate clothing in the laboratory at all times. You MUST wear your safety goggles over your eyes at all times in the laboratory! DO NOT wear loose or skimpy clothing (saris, neckties, shorts, halter-tops, overly large or ragged laboratory coats). DO wear closed shoes in the lab. DO NOT wear sandals or perforated shoes. Long hair must be tied back. file:///D|/Chem234/Syllabus/Safty.htm (6 of 7) [1/13/2001 10:41:59 AM] LABORATORY SAFETY file:///D|/Chem234/Syllabus/Safty.htm (7 of 7) [1/13/2001 10:41:59 AM] Chem 234 Check-In Procedure Chem 234 Check-In Procedure You will not be charged for stocking your drawer during this initial check of its contents. After this, however, you will be char ed for replacing any damaged or missing items. You MUST have the contents of your locker checked by a TA or a storeroom manager upon your termination of enrollment in Chem 234 or your completion of this course. Failure to have your locker checked will result in a fine of ten dollars. This fine will be in addition to the charges that will be made for replacing any broken or missing equipment. Finally, you are responsible for the cleanliness of your own work area. For safety reasons, keep it clean! 1. Remove the equipment in your drawer, and check it against the Equipment List on the last page of the syllabus. 2. Return any extra equipment you find in your drawer to the storeroom manager in 469 Noyes Lab. 3. Mark on the Equipment List any items you find broken or missing. Remember to check for "star" cracks in glassware. Make sure that your thermometer is not cracked, and that the 0-ring is inside the thermometer adapter. Also check to see that the four indentions at the bottom of your distilling column are intact. 4. Bring your completed Equipment List to the storeroom manager in room 469 Noyes Lab. Also bring him any repairable damaged equipment for replacement. He will replace your damaged equipment with good items and will provide you with any items you have found missing. Your drawer should now have a full set of equipment as listed on the Equipment List. 5. Return all checked items to your drawer. Matches, rubber bulbs, and glass stirring rods should stay in your drawer. 6. Leave the Bunsen burner on the top of your desk. Leave the Thiele tube in the clamp provided for it above your work area. DO NOT put the Bunsen burner, Thiele tube, or vacuum hoses in your locker. All ironware is to be kept in the bins at the ends of the lab. If you use these items during a laboratory session, remember to return them to the proper bins at the end of the laboratory session. DO NOT put them in your locker. All tubing (except that on the steam bath or Bunsen burner) and wire gauze should be kept in the boxes at the east and west ends of the lab. To identify the names of equipment consult the display just inside the door or the equipment list shown below. file:///D|/Chem234/locker/Checkin.htm (1 of 7) [1/13/2001 10:43:53 AM] Chem 234 Check-In Procedure Chemistry 234 equipment display in 464 Noyes Lab. Chem. 234 Equipment List 1 Adapter, Bent, 105° 14/20standard taper joint 1 Adapter, Distilling, 14/20, w/10/18 Thermometer joint 1 Adapter, Teflon, Thermometer,10/18 w/ O-ring 2 Beaker, Griffin, w/spout, 30 mL file:///D|/Chem234/locker/Checkin.htm (2 of 7) [1/13/2001 10:43:53 AM] Chem 234 Check-In Procedure 1 Beaker, Griffin, w/spout, 50 mL 1 Beaker, Griff in, w/spout, 100 mL 1 Beaker, Griff in, w/spout, 150 mL 1 Beaker, Griffin, w/spout, 250 mL 1 Beaker, Griff in, w/spout, 400 mL 1 Bulb, pipette, pure gum rubber 2 Clamps, i.e., Plastic, yellow, for 14/20 joint 2 Clips, Tubing, Steel (used to attach gum rubber hoses to glass joints) 1 Column, Distilling, 14/20 standard taper joint.,10 x 130 mm, 1 Condensor, Leibig, 14/20 standard taper joint file:///D|/Chem234/locker/Checkin.htm (3 of 7) [1/13/2001 10:43:53 AM] Chem 234 Check-In Procedure 1 Cylinder, pyrex glass, graduated, 10 mL 1 Cylinder, poly, graduated, 50 mL 2 Flask, Erlenmeyer, 25 mL 2 Flask, Erlenmeyer, 50 mL 2 Flask, Erlenmeyer, 125 mL 1 Flask, Erlenmeyer, 250 mL file:///D|/Chem234/locker/Checkin.htm (4 of 7) [1/13/2001 10:43:53 AM] Chem 234 Check-In Procedure 1 Flask, Filter, 125 mL& 1 Flask, Pear-shaped, 14/20 standard taper joint, 25 mL 1 Flask, Recovery, 14/20 standard taper joint, 25 mL 1 Flask, Recovery, 14/20 standard taper joint, 50 mL 1 Flask, Recovery, 14/20 standard taper joint, 100 mL 1 Funnel, Buchner, Porcelain, #2, 67 mm 01); Includes one rubber stopper w/ center hole 1 Funnel, Hirsch, Porcelain, 4/0, 55 mm OD file:///D|/Chem234/locker/Checkin.htm (5 of 7) [1/13/2001 10:43:53 AM] Chem 234 Check-In Procedure 1 Funnel, Polyethylene, 31, 4" x 4" 1 Funnel, Powder, P.E., 65 x 15 mm 1 Funnel, Separatory, 250 mL,- Includes one each #22 glass stopper & teflon stopcock. (Note: TefIon stopcock has two O-rings. One black rubber O-ring & one white teflon O-ring) 1 Glass rod, 9mm x 255mm 1 Holder, Test Tube 1 Rack, Test Tube 1 Spatula, Stainless Steel, double bladed 1 Stopper, Glass, 14/20 standard taper joint. 6 Test Tube, Pyrex, 13 x 100 mm 3 Test Tube, Pyrex, 25 x 150 Mm 1 Test Tube, Pyrex, 25 x 200 mm 1 Test tube, w/Side-arm, 25 x 200 mm 1 Thermometer, -100 to 260&degC 1 Tube, Drying, Polyethylene file:///D|/Chem234/locker/Checkin.htm (6 of 7) [1/13/2001 10:43:53 AM] Chem 234 Check-In Procedure 1 Wash bottle, Polyethylene, 250 mL 1 Watch Glass, Plain, 75 mm diameter file:///D|/Chem234/locker/Checkin.htm (7 of 7) [1/13/2001 10:43:53 AM] Experiment 1 Experiment 1 Melting Points (10 points) I. Summary Melting points are very useful in determining the purity of a compound. Throughout this class you will measure the melting point of your products in order to assess purity. You will practice this technique on a sample of benzoic acid. Put open end of tube in the sample. Tape tube so sample falls to the bottom of the tube. Impure samples have lower melting points than pure solids. You can use this information to determine the identity of an unknown through the mixed melting point method. You will be given an unknown from the list below. By determining the melting point of your unknown, you will be able to narrow the choices to three, based on the melting point ranges. You will then mix your unknown with EACH of the three possibilities. You can determine the melting point of each of the mixtures. In the two mixtures that contain two different compounds, you should see a drastic change in the melting point since the samples are not pure. One of the samples will contain only one compound, and this should be reflected by the melting point. Compound file:///D|/Chem234/Exp1/Experiment1.htm (1 of 4) [1/15/2001 7:03:11 AM] Melting Point Experiment 1 acetanilide p-aminobenzoic acid camphoric acid trans -cinnamic acid malonic acid p-nitrophenol resorcinol succinic acid urea 113-115&degC 188-189&degC 183-186&degC 133-134&degC 135-137&degC 113-115&degC 110-113&degC 187-189&degC 133-135&degC First obtain a sample of benzoic acid from your TA (about a spatula-tip). Grind the sample into a fine powder, and take two melting points, one fast, one slow (2 &degC/min). Obtain your unknown from the stockroom, and grind this sample if necessary. Take two melting points of your known (one fast, one slow). You should now be able to determine the three possibilities for the identity of your unknown. Mix a spatula-tip of your unknown with a spatula-tip of each of the three possible compounds. These samples should be well mixed to ensure the best results. Take the melting points of each of the mixtures, and use this information to determine the identity of your unknown, Samples of two compounds for mixed melting point. Grind samples together. file:///D|/Chem234/Exp1/Experiment1.htm (2 of 4) [1/15/2001 7:03:11 AM] Experiment 1 III. Report Your report should include the following: 1. Reference to the procedure with any changes noted 2. Data for melting points of benzoic acid 3. Data for melting points of unknown sample 4. Data for the three mixtures 5. Identity of your unknown 6. Interpretation of data and a short conclusion You should measure the melting point with the Thiele tube at your bench or the electrically heated melting point apparatus in the lab. Thiele tube melting point apparatus file:///D|/Chem234/Exp1/Experiment1.htm (3 of 4) [1/15/2001 7:03:11 AM] Experiment 1 Electrically heated melting point apparatus You should complete the web-based ChemNet tutorials on melting points before comming to the lab. file:///D|/Chem234/Exp1/Experiment1.htm (4 of 4) [1/15/2001 7:03:11 AM] Experiment 2 Experiment 2 Recrystallization (10 points) I. Summary You will be learning several techniques for purification of organic compounds. One very effective way to purify solids is through recrystallization. In this experiment, you will be given an impure sample of benzoic acid that you will purify by recrystallization. You will also detennine the melting point of the recrystallized benzoic acid to assess the purity. Impure sample of benzoic acid. Benzoic acid after recrystallization. II. Procedure The procedure for recrystallization is found in your text, page 97. You will make the following changes to the procedure: 1. Scale up the procedure so that it accommodates 2 grams of benzoic acid. 2. Weigh the sample of impure benzoic acid before you begin recrystallization. 3. Skip the "Decoloration" section. file:///D|/Chem234/Exp2/Experiment2.htm (1 of 5) [1/15/2001 7:04:00 AM] Experiment 2 Heat the sample with enough water to dissolve all soluble material. Add more water if needed. file:///D|/Chem234/Exp2/Experiment2.htm (2 of 5) [1/15/2001 7:04:00 AM] Experiment 2 Filter the hot solution to remove insoluble material Boil off some of the water. Use a boiling stick when you boil off the solvent. file:///D|/Chem234/Exp2/Experiment2.htm (3 of 5) [1/15/2001 7:04:00 AM] Experiment 2 Be sure to use the GLASS powder funnel for your hot filtration rather than the plastic funnel. Crystals form as solution is cooled. Cool to complete crystallization. file:///D|/Chem234/Exp2/Experiment2.htm (4 of 5) [1/15/2001 7:04:00 AM] Experiment 2 Filter, wash, and dry the product. Note: You will need a total of 40-5O mL of water for this experiment. If you add too much water you can boil some off after filtration. III. Report Requirements Your lab report should include the following: 1 . References to procedure with any changes noted 2. Weight of recrystallized benzoic acid 3. Percent recovery of benzoic acid 4. Melting point of recrystallized benzoic acid 5. Interpretation of data and short conclusion (discuss purity, recovery, and overall effectiveness of recrystallization) file:///D|/Chem234/Exp2/Experiment2.htm (5 of 5) [1/15/2001 7:04:00 AM] Experiment 3 Experiment 3 Fractional Distillation (15 points) I. Summary Fractional distillation is a technique used to separate volatile compounds with boiling points that differ by less than 50 OC. In this experiment, you will distill a mixture of methanol (b.p. 65&degC and water (b.p. 100&degC). Using data obtained in this experiment, you will graphically demonstrate how head temperature changes with the composition of the distillate. II. Procedure Obtain 60 mL of a sample of one of the methanol/water mixtures from the carboys in the lab. Record which sample you chose (A, B, or Q. Add the mixture and a few boiling chips to a clean 100 mL round bottom flask. Set up the apparatus for fractional distillation as demonstrated in lab. Use the Thermowell to heat the flask. Plug it into the Variac on your bench. Do not plug directly in the wall outlet. file:///D|/Chem234/Exp3/Experiment3.htm (1 of 4) [1/15/2001 7:04:45 AM] Experiment 3 Use a distilling column to help seperate the mixture. The thermometer adapter allows you to put the thermometer into the distilling adapter. file:///D|/Chem234/Exp3/Experiment3.htm (2 of 4) [1/15/2001 7:04:45 AM] Experiment 3 Use joint clamps and hose clamps. Here is the completed apparatus. Have your set-up approved by your TA before you begin. Begin heating the sample on a low setting and increase the temperature as needed. Collect the distillate in a graduated cylinder. Record the head temperature when you collect the first drop of distillate, and then record the head temperature at every two milliliters of distillate collected. Stop the distillation when you have recorded three reading at about 100&degC, even if you haven't distilled all of the sample. file:///D|/Chem234/Exp3/Experiment3.htm (3 of 4) [1/15/2001 7:04:45 AM] Experiment 3 DO NOT heat the flask to dryness. III. Report Your report should include the following: 1 . References to procedure with changes noted 2. Table of recorded data (volume collected and head temperature) 3. Graph of distillation data: Make a plot with the head temperature on the y-axis and the volume of distillate on the x-axis (see page 125 of your text). Draw an AVERAGE line through the data points. DO NOT simply connect the dots. 4. Summary table of data: Use your graph to determine the volume and boiling point range of each "fraction". Make a table that includes Fraction number, collected volume, and boiling range. 5. Interpretation of data and conclusion, including the methanol/water ratio of your sample IV. TUTORIALS You should work the web-based ChemNet tutorials on distillation before going to the lab. file:///D|/Chem234/Exp3/Experiment3.htm (4 of 4) [1/15/2001 7:04:45 AM] Experiment 4 Experiment 4 Extraction (20 points) I. Summary Extraction is a technique used to separate and/or purify compounds. In this experiment, you will be separating a mixture of anthracene, p-nitroaniline, and benzoic acid into the individual components based on their differing solubilities in immiscible phases. Mixture of anthracene, p-nitroaniline, and benzoic acid. Purified benzoic acid file:///D|/Chem234/Exp4/Experiment4.htm (1 of 7) [1/15/2001 7:33:56 AM] Experiment 4 Purified anthracene Purified p-nitroaniline II. Prelab BEFORE class, prepare a flow chart that outlines every step of the experiment. The flow chart should show a branch point at every extraction step (including isolation of the three compounds), and you should note which compounds are in each layer at each step. You must have this flow chart completed before you will be allowed to begin the experiment,and the flow chart will be handed in with your lab report. III. Procedure Obtain a mixture of benzoic acid, p-nitroaniline, and anthracene. Weigh the mixture, and then dissolve the mixture in 75 mI, of methylene chloride. If some solid doesn't dissolve after several minutes of stirring, filter the solution. Add the solution to the separatory funnel and proceed through the following steps: file:///D|/Chem234/Exp4/Experiment4.htm (2 of 7) [1/15/2001 7:33:56 AM] Experiment 4 First Period: 1 . Add 50 mL of 3M HCI to the separatory funnel. Shake and vent the funnel as described in the textbook readings. Be sure to vent the separatory funnel to avoid pressure build up. If you get hydrochloric acid on your skin be sure to wash with large amounts of water. Contact your TA for assistance. file:///D|/Chem234/Exp4/Experiment4.htm (3 of 7) [1/15/2001 7:33:56 AM] Experiment 4 2. Allow the layers to separate in the funnel. Drain the bottom layer into a flask labeled "organic," and pour the top layer into a flask labeled "acidic extract" . 3. Return the organic layer to the separatory funnel, and repeat steps 1 and 2. Add the top layer file:///D|/Chem234/Exp4/Experiment4.htm (4 of 7) [1/15/2001 7:33:56 AM] Experiment 4 from this extraction to the flask labeled "acidic extract". 4. Place the organic extract into the separatory funnel, and add 50 mL of 3M NaOH. Shake and vent the separatory funnel as before. If you get sodium hydroxide solution on your skin be sure to wash with large amounts of water. Contact your TA for help. 5. Allow the layers to separate. Drain the bottom layer into a flask labeled "organic," and pour the top layer into a flask labeled "basic extract." 6. Return the organic layer to the separatory funnel, and repeat steps 4 and 5. Add the top layer from this extraction to the flask labeled "basic extract." 7. Cool the ACIDIC EXTRACT in an ice bath. Basify the ACIDIC EXTRACT with 6M NaOH (calculate how much you need). Check the pH of the solution with pH paper to make sure it is BASIC. Filter the resulting precipitate by vacuum filtration. Place the solid on a piece of filter paper, and allow it to air dry until the next lab period. USE CAUTION IN HANDLING THE SODIUM HYDROXIDE. 8. Cool the BASIC EXTRACT in an ice bath. Acidify the BASIC EXTRACT with 6M HCl. Check the pH of the solution with pH paper to make sure it is acidic. Collect the resulting solid by vacuum filtration. Place the solid on a piece of filter paper and allow it to air dry until the next lab period. 9. To the ORGANIC layer, add a few spatula tips of Na2S04 to dry the organic layer. Allow the solution to stand for 10 minutes. 10. Filter the solution through fluted filter paper into an Erlenmeyer flask. Evaporate the methylene chloride using the apparatus that will be demonstrated in lab. file:///D|/Chem234/Exp4/Experiment4.htm (5 of 7) [1/15/2001 7:33:56 AM] Experiment 4 Second Period: 1. Obtain weights of benzoic acid, p-nitroaniline, and anthracene and melting points of benzoic acid and p-nitroaniline. 2. Place all solids in tared, labeled vials, and give them to your TA. III. Report Requirements Your report should contain the following: I . Reference to procedure with changes noted 2. Flow chart described above 3. Weights of all three solids 4. Melting points of benzoic acid and p-nitroaniline (both weights and melting points should be reported in tabular form) file:///D|/Chem234/Exp4/Experiment4.htm (6 of 7) [1/15/2001 7:33:56 AM] Experiment 4 5. Interpretation of data and conclusion -How pure are the extracted compounds? -How could you purify them further? -What was the composition of the original mixture, i.e., the ratio of benzoic acid: p-nitroaniline: anthracene? - recovery file:///D|/Chem234/Exp4/Experiment4.htm (7 of 7) [1/15/2001 7:33:56 AM] Experiment 5 Experiment 5 Preparation of 2-chloro-2-methylpropane (20 points) I. Summary In the experiment, you will be preparing 2-chloro-2-methylpropane from 2-methyl-2-propanol via an SN1 reaction. II. Procedure You will follow the procedure from the text (pages 389-390). Note that you will be using 2-methyl-2-propanol instead of 1-methyl-2-butanol. However, all other reagents remain the same. You should double all quantities listed in the procedure. Shake the t-butyl alcohol with HCl. Be sure to vent the separately funnel to avoid pressure build up. If you get hydrochloric acid on your skin wash immediately with large amounts of water. Contact your TA for assistance. Separate, wash, and dry the t-butyl chloride. file:///D|/Chem234/Exp5/Experiment5.htm (1 of 4) [1/15/2001 7:11:17 AM] Experiment 5 The crude t-butyl chloride is washed to remove excess acid. Add the sodium bicarbonate solution slowly to avoid rapid formation of CO2 as shown here. Setup for distillation of the t-butyl chloride. Collect the product in a cooled, tared container. file:///D|/Chem234/Exp5/Experiment5.htm (2 of 4) [1/15/2001 7:11:17 AM] Experiment 5 Use joint clamps on the condenser and hose clamps on the water lines. III. Post-lab Questions 1. Washing the crude 2-chloro-2-methylpropane with aqueous sodium bicarbonate resulted in gas evolution. Write a balanced equation for this reaction. 2. Could anhydrous NaOH be used to dry the 2-chloro-2-methylpropane instead of Na2SO4? 3. Explain why 1-butanol does not react with HC1 under the conditions used in this experiment. IV. Report Requirements Your report should contain the following: 1 . Reference to the procedure with changes noted 2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles. 3. Mechanism of the reaction: use arrows to show electron movement; include all intermediates and side reactions 4. Calculation of the theoretical yield 5. Weight of product 6. Boiling range of the product 7. Interpretation of data and conclusion file:///D|/Chem234/Exp5/Experiment5.htm (3 of 4) [1/15/2001 7:11:17 AM] Experiment 5 8. Answers to post-lab questions file:///D|/Chem234/Exp5/Experiment5.htm (4 of 4) [1/15/2001 7:11:17 AM] Experiment 6 Experiment 6 Preparation of methylbenzoate (40 points) I. Summary In this experiment you will prepare methylbenzoate by reacting benzoic acid with methanol using sulfuric acid as a catalyst. Since this is a reversible reaction, it will reach an equilibrium that is described by the equilibrium constant, Keq . In this experiment, you will isolate the product, methyl benzoate and any unreacted benzoic acid. Using this data, you will calculate the equilibrium constant for the reaction as described in part III. II. Procedure First Period Obtain a 10g (0.082 mol) sample of benzoic acid from the storeroom (weigh the benzoic acid to obtain an exact weight before you begin). Add to it 0.62 mol of methanol in a 100 mL round-bottomed flask. Cautiously, add 3 mL concentrated H2S04 down the side of the flask. After gently swirling the contents of the flask, attach a reflux condenser and reflux the mixture for about 60 min. Sulfuric acid causes acid burns on the skin. If you get some on your skin be sure to wash with large amounts of water. Contact your TA! Allow the solution to cool. Add 50 mL of water to a separatory funnel, then add the contents of the flask. The flask should then be washed with 40 mL of dichloromethane (CH2C2) and the washings transferred file:///D|/Chem234/Exp6/Experiment6.htm (1 of 5) [1/15/2001 7:12:06 AM] Experiment 6 to the separatory funnel. Use the separatory funnel to extract the ester into the CH2Cl2 (organic) layer. All sequentially with 25 mL water and 25 mL 0.6M sodium bicarbonate* (Caution, CO2 will be given off with effervescence). After shaking and venting the funnel, remove the sodium bicarbonate layer and test to see if it is basic. Then wash the organic layer with NaCl (salt) solution, separate the organic layer and dry with anhydrous magnesium sulfate. Remove theMgS04by gravity filtration, and concentrate the solution using the same apparatus used to remove CH2Cl2 from anthracene in Experiment 4. *The aqueous layer from the sodium bicarbonate wash should be acidified with concentrated HCl The unreacted benzoic acid should precipitate. Remove the solvent by vacuum filtration. Collect and dry the solid benzoic acid and let it air-dry in your drawer until the next lab period. Second Period Distill the crude methyl benzoate (b.p. 199°), collecting anything that boils between 170&degC and 200&degC. Weigh the sample of methyl benzoate and determine the yield. Weigh the sample of recovered benzoic acid. Place both samples in labeled vials, and give them to your TA. Caution: 1. Methanol is flammable and toxic 2. Dichloromethane is toxic 3. Handle concentrated H2SO4 and HCl with great care 4. Vent the separatory funnel frequently after swirling and subsequent shaking. CO2, gas is evolved and will build up pressure in the funnel. file:///D|/Chem234/Exp6/Experiment6.htm (2 of 5) [1/15/2001 7:12:06 AM] Experiment 6 III. Calculation of the Equilibrium Constant, Keq. Calculate a value for the equilibrium constant Keqbased upon your weight of recovered benzoic acid. In addition, calculate the percent yield of your synthesis based upon the amount of starting benzoic acid used, and another percent yield based upon the amount of benzoic acid that actually reacted (as determined by the weight of recovered benzoic acid). Calculation of the Equilibrium Constant Keq: Since the volume of the reaction mixture is the same for each component, we can use moles instead of molarities as the units in our Keq expression. Let Bi = initial moles of benzoic acid, let Mi = initial moles of methanol, and let X = final moles of methyl benzoate produced at equilibrium. Based upon the stoichiometry of equation 1, we can conclude three things about X, namely that X = final moles of water produced at equilibrium, X = moles of benzoic acid consumed when equilibrium is established, and X = moles of methanol consumed when equilibrium is established. Using our definitions, equation 2 becomes: We know Bi and Mi. All we need to do before we can calculate Keq is to Put X in terms of something known, like the amount of benzoic acid recovered. Let Bf = final moles of benzoic acid present at equilibrium. Thus Bf = Bi - X, or X = Bi - Bf. Substituting(Bi - Bf) for X in equation 3 gives: We can now calculate Keq by entering the appropriate values of the moles of benzoic acid used (Bi), the moles of methanol used (Mi), and the moles of benzoic acid recovered (Bf) into equation 5. file:///D|/Chem234/Exp6/Experiment6.htm (3 of 5) [1/15/2001 7:12:06 AM] Experiment 6 Calculation of the % Yield based upon initial amount of benzoic acid used: Let E = moles of methyl benzoate ester obtained. Note that this calculation is valid only if benzoic acid is the limiting reagent. Calculation of the % Yield based upon amount of benzoic acid that is consumed: IV. Post-lab Questions 1. How is sulfuric acid involved in the formation of methyl benzoate? 2. Write a balanced equation for the formation of ethyl acetate from ethanol and acetic acid. 3. Using K=3.0, calculate the number of moles of methyl benzoate which could be formed from 0. 1 mol of benzoic acid and 0.3 mol of methanol. V. Report Requirements Your lab report should contain the following information: 1 . Reference to procedure with any changes noted 2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles. 3. The complete mechanism showing all intermediates and arrows to demonstrate electron movement 4. Flow chart for the isolation of methyl benzoate and unreacted benzoic acid 5. Weight of recovered benzoic acid file:///D|/Chem234/Exp6/Experiment6.htm (4 of 5) [1/15/2001 7:12:06 AM] Experiment 6 6. Weight and boiling point of methyl benzoate 7. Calculations a. percent yield based on amount of benzoic acid with which you started b. percent yield based on amount of benzoic acid consumed c. equilibrium constant 8. Interpretation of data and conclusion 9. Answers to post-lab questions file:///D|/Chem234/Exp6/Experiment6.htm (5 of 5) [1/15/2001 7:12:06 AM] Experiment 7 Experiment 7 Preparation of methyl-m-nitrobenzoate (25 points) I. Summary In this experiment, you will synthesize methyl-m-nitrobenzoate from methyl benzoate via electrophilic aromatic substitution. The isolated product will be purified by recrystallization, and purity will be determined from the melting point. II. Procedure Bring a clean, dry 25 x 150 mm test tube to the storeroom manager and trade it in for a 6.2 mL (6.8 g, 0.050 moles) sample of methyl benzoate (weigh the sample before you begin to obtain an exact weight). Place 14.5 mL (26.7 g) of concentrated sulfuric acid in a 125 rnL Erlenmeyer flask, cool it to 0 *C in an ice/water bath, then add all of the methyl benzoate with swirling. Separately, prepare a mixture of 5.0 ml- (9.2 g, 0.094 moles) of concentrated sulfuric acid and 5.0 mL (7.1 g, 0. 113 moles) of concentrated nitric acid, cool this mixture by adding the acids in a vessel immersed in an ice bath. Be very careful in handling the concentrated sulfuric acid and nitric acid. If you get acid on you be sure to wash with large amounts of water and contact your TA immediately for assistance. file:///D|/Chem234/Exp7/Experiment7.htm (1 of 4) [1/15/2001 7:13:18 AM] Experiment 7 When it has cooled, add this cold acid mixture dropwise over 5 minutes to the methyl benzoate solution, which is kept swirling in the ice bath. After the addition is complete, allow the reaction mixture to stand at room temperature for an additional 10 minutes, swirling it occasionally. file:///D|/Chem234/Exp7/Experiment7.htm (2 of 4) [1/15/2001 7:13:18 AM] Experiment 7 Pour the mixture, with stirring, over about 150 cc of crushed ice. Collect the solid precipitate by suction filtration, and wash it thoroughly with water to remove any traces of acid. Finally, wash the product twice with 5 mL portions of ice-cold methanol. Recrystallize your product from a small amount of methanol. Collect the crystals by vacuum filtration, and allow the product to air dry in your drawer until the next lab period. Obtain the weight and melting point of your dry product. Give your sample to your TA. file:///D|/Chem234/Exp7/Experiment7.htm (3 of 4) [1/15/2001 7:13:18 AM] Experiment 7 III. Post-lab Questions 1. Draw all resonance structures for the nitration of methyl benzoate occurring at the ortho, meta, and para positions. Be sure to include all charges. 2. Circle any resonance structures which demonstrate why the ortho-para positions are unfavorable sites for nitration. IV. Report Requirements Your report should contain the following information: 1 . Reference to the procedure with changes noted 2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles 3. Mechanism of the reactions showing the structures of intermediates and arrows to demonstrate electron movement 4. Experimental yield and percent yield (show calculations) 5. Color of product 6. Melting point of product 7. Interpretation of data and conclusion 8. Answers to post-lab questions file:///D|/Chem234/Exp7/Experiment7.htm (4 of 4) [1/15/2001 7:13:18 AM] Cyclohexene Experiment Experiment 8 Dehydration of Cyclohexanol I. Summary In this experiment, you will synthesize cyclohexene via acid-catalyzed dehydration of cyclohexanol. This reaction will be carried out by heating the components and collecting impure product. A second distillation will be necessary to purify cyclohexene. Purity will be determined by the boiling point of the collected product. The product will be tested for unsaturation using the Baeyer test. II. Procedure Bring a clean, dry 25 x 150 mm test tube to the storeroom manager, and trade it for a sample of cyclohexanol. Obtain a weight or volume for the sample (around 14.2g or 15 mL). Be sure to use the mass of your sample for all of your calculations. Into a 50 mL round bottom flask, add the cyclohexanol, 9 M sulfuric acid (7.5 mL), and a few boiling chips. Mix the contents of the flask with gentle swirling. Equip the flask for fractional distillation (see experiment 3). Use a heating mantle with sand to heat the distillation flask. file:///D|/Chem234/Cyclohexene/cyclohexene.htm (1 of 3) [1/15/2001 7:14:11 AM] Cyclohexene Experiment Use an erlenmeyer flask as the collection vessel, and cool it in an ice bath during the distillation. Heat the reaction mixture, and collect all distillates while maintaining a head temperature in the range of 80-85 °C. Discontinue the reaction once about 5 mL remains in the distillation flask. Add several spatula-tips full of potassium carbonate to the crude product in the Erlenmeyer flask. Swirl the mixture occasionally over a 10-15 minute period, and add more potassium carbonate IF the liquid remains cloudy. Thorough drying is very important (why?).Transfer the product to an 25 mL round bottomed flask by decanting or using a pipet. Add a few boiling stones to the flask, and purify the product by simple distillation. Use a tared vial as a receiver, and cool the receiver in an ice bath. Collect distillate with a head temperature between 80-85 °C. Obtain a weight of your purified product. Note: If product is cloudy, add several spatula-tips full of potassium carbonate to the vial, and transfer the dried product to another tared vial by pipet before weighing. Bayer Test for Unsaturation: To test your product for unsaturation, follow the procedure on pages 653-654 of your lab text for the file:///D|/Chem234/Cyclohexene/cyclohexene.htm (2 of 3) [1/15/2001 7:14:11 AM] Cyclohexene Experiment Baeyer test. Perform both the test and the blank. III. Report Requirements Your report should contain the following information: 1. Reference to procedure with changes noted. 2. Equation of the reaction showing structures and products. Below each reactant, list molecular weight, amount used in grams (or mL) and moles. Below each product list the molecular weight and theoretical yield in grams and moles. 3. Mechanism of the reaction showing structures of intermediates and arrows to demonstrate electron movement. 4. Experimental yield and percent yield (show calculations) 5. Weight of product. 6. Boiling point range of product. 7. For the Baeyer test, include both the mechanism of the reaction and your results for cyclohexene and the blank. 8. Interpretation of data and conclusions file:///D|/Chem234/Cyclohexene/cyclohexene.htm (3 of 3) [1/15/2001 7:14:11 AM] Experiment 8 Experiment 9 Preparation of trans -p -Anisalacetophenone (25 points) I. Summary In this experiment, you will be preparing trans-p-anisalacetophenone (m.p. 77-78°) from p-anisaldehyde and acetophenone via a crossed aldol condensation. The product will be purified by recrystallization, and purity will be assessed by taking the melting point of the product. II. Procedure Follow the procedure found on pages 499-500 in your text (omit the "Analysis" portion of the procedure. You will need to scale the procedure to six times that listed in the text. Take a clean, dry 25 x 150 mm. test tube to the storeroom manager and trade it in for a sample of p-anisaldehyde. Weigh the sample before you begin the experiment, and adjust the amount of acetophenone accordingly. Once you have obtained the recrystallized product, allow the product to air-dry in your drawer until the next lab period. Obtain the weight and melting point. file:///D|/Chem234/Exp9Aldol/Experiment8.htm (1 of 5) [1/15/2001 7:17:02 AM] Experiment 8 Locate starting material Measure reactants file:///D|/Chem234/Exp9Aldol/Experiment8.htm (2 of 5) [1/15/2001 7:17:02 AM] Experiment 8 Mix Let stand file:///D|/Chem234/Exp9Aldol/Experiment8.htm (3 of 5) [1/15/2001 7:17:02 AM] Experiment 8 Filter crude product Recrystallize Purified product III. Report Requirements Your report should contain the following: 1. Reference to procedure with changes noted. file:///D|/Chem234/Exp9Aldol/Experiment8.htm (4 of 5) [1/15/2001 7:17:02 AM] Experiment 8 2. Equation of the reaction showing structures of reactants and products. Below each reactant, list the molecular weight, and amount used in grams (or mLs) and moles. Below each product, list the theoretical yield in grams and moles. 3. Mechanism of the reactions showing the structures of intermediates and arrows to demonstrate electron movement 4. Mass of product 5. Percent yield (show calculations) 6. Melting point of product (include literature melting point) 7. Interpretation of data and conclusion, including detailed error analysis file:///D|/Chem234/Exp9Aldol/Experiment8.htm (5 of 5) [1/15/2001 7:17:02 AM] Experiment 10 Experiment 10 Identification of an Aldehyde, Amine, Alcohol, or Ketone (45 points) I. Summary In this experiment, you will be given an unknown that is either an aldehyde, ketone, amine, or alcohol. The possible unknowns are listed in tables that will be posted in the laboratory. You will determine the boiling point of your unknown, and using the NMR and IR spectra,classification tests, and melting point of the derivative, you will determine the identity of your unknown. II. Procedure Bring a clean, dry 13 x 100 mm test tube to the storeroom manager and trade it for a sample of your unknown (approximately 2.5g). Your TA will give you copies of the 1H and 13C NMR and IR spectra for your unknown. You should then do the following: 1. Determine the boiling or melting point of the unknown. To determine the boiling point, set up an apparatus for simple distillation. In this experiment, however, you will not actually distill your unknown, so pay careful attention to the following description. Use your 25mL pear-shaped flask as a distillation flask. You will actually insert the thermometer all the way into the flask, so add enough of your unknown to the distillation flask to completely cover the mercury bulb of your thermometer. Apparatus for measuring the boiling point of your unknown. Heat the flask until the unknown boils. You should record the boiling point as the temperature at which the thermometer stabilizes. To obtain a boiling (or melting) point range, subtract 5&degC from the observed temperature to get a lower limit, and add 10° to the observed temperature to get an upper limit. Report this range to your TA who will tell you if the boiling point or melting point of your unknown is within this range. 2. Determine the functional group present in your unknown using the IR spectrum. BEFORE you come file:///D|/Chem234/Exp10/Experiment10.htm (1 of 3) [1/15/2001 7:18:01 AM] Experiment 10 to class, know the stretching frequencies for carbonyls, O-H, and N-H. By looking at the IR spectrum, you will be able to determine which of these functional groups is present in your unknown. This will determine which tests you should perform on your unknown. Check your conclusions with your TA before you begin the tests. 3. Perform characterization tests on your unknowns. All of the reagents will be prepared ahead of time for your use. You only need to do those tests required for your functional group. Aldehydes/ketones: 2,4-DNP (642-643), Tollens (644-645),iodoform(647-649) alcohols: chromic acid (646-647), iodoform. (647-649) amines: Hinsberg (672-675), HCl solubility (628) 4. Narrow your choices. Find the tables for your functional group and write down all of the compounds in that table whose boiling/melting points fall within the range determined in step 1. Your unknown will be one of these compounds. 5. Synthesize a derivative. Once you have determined the possible identities of your unknown, you will need to make a derivative. The type of derivative you make will be determined by the functional group. Choose the derivative that you think will give you the most useful information. You should recrystallize your solid carefully, since an impure derivative would give you a low melting point which would be misleading. aldehydes/ketones: 2,4-DNP (642-643), semicarbazone (650) alcohols: 3,5-dinitrobenzoate (665, Method A; see experiment 9 for amounts of reagents) amines: benzenesulfonamides (678), benzamides (677-678) 6. Use your NMR spectra to help you determine the identity of your unknown. You should interpret the spectra to the best of your ability. For each set of peaks in the 1H NMR, you should determine chemical shift, integration, and multiplicity. For each peak in the 13C NMR, you should determine the chemical shift. See your text for a full description of how to use this information to determine the structure of your compound. 7. Interpret the data in lab, and try to determine the identity of your unknown before you leave the lab. At least narrow your list to a maximum of five possible compounds. III. Lab report requirements Your lab report (due the day scheduled for check-out) should contain the following: 1 . Reference to the procedure of each chemical test and derivative 2. Melting point or boiling point of your unknown 3. Results of each chemical test and your interpretation of these results 4. Data table for 1H NMR: For each set of peaks, give the chemical shift, splitting pattern integration, and your assignment of which proton of set of protons in the compound give rise to the file:///D|/Chem234/Exp10/Experiment10.htm (2 of 3) [1/15/2001 7:18:01 AM] Experiment 10 peak or set of peaks. 5. Data table for 13C NMR: For each peak, report the chemical shift and assignment. 6. Data table for IR: (assign bands above 1500 cm-1) For each band, report position in wavenumbers, a description of the band (strong, weak, or medium, sharp or broad) and your interpretation. 7. Preparation of the derivative (type of derivative and reference to procedure) 8. Melting point of the derivative 9. Identification of the compound with an explanation of how you arrived at this conclusion file:///D|/Chem234/Exp10/Experiment10.htm (3 of 3) [1/15/2001 7:18:01 AM] VII Chem 234 Check-Out Procedure Any student who fails to be checked out of the lab by a TA or by a storeroom manager will be charged with a fine of ten dollars. This fine is assessed in addition to the charges that are made to replace any broken or missing equipment. 1. Remove all equipment from your drawer and wash each item with Tetrox and water. 2. Place new blotter paper lining in your drawer. 3. Check your equipment against the Equipment List. Return any extra equipment to the storeroom and replace any missing or broken items with new ones from the storeroom. 4. Place all ironware (except the metal stand) in the bins at the ends of the lab. 5. Place all tubing (except that on the steam baths and Bunsen burners) and wire gauze in their proper storage boxes located at the east and west ends of the lab. 6. All matches, rubber bulbs, and stirring rods should be left in the drawer. 7. Leave the rubber stoppers on the Hirsch and Buchner funnels. Throw out any cork stoppers. 8. Make sure that the four indentations at the bottom of your distilling column are intact. 9. Make sure that your thermometer is not cracked and that the O-ring is still inside the thermometer adapter. 10. Remove the calcium chloride from inside the drying tube. 11. Remove any stopcocks and stoppers from the separatory funnels. Tie them to the funnels with string. 12. Place all the cleaned equipment back into your drawer. Your drawer should now have a full set of equipment as listed on the Equipment List. 13. Sign the equipment list (pg. 24) and have your TA sign the equipment list. Take this list to Mike Eubanks (room 469) to receive your breakage card. file:///D|/Chem234/Checkout/Checkout.htm [1/15/2001 7:41:12 AM]