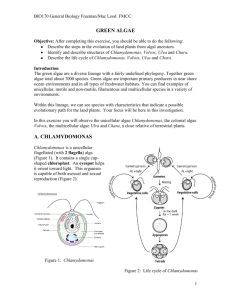
Preliminary investigation into the induction of reproduction in Ulva spp
advertisement

Preliminary investigation into the induction of reproduction in Ulva spp. in Southeast Queensland for mass cultivation purposes Prue Pettett B.Sc. (Environmental Science) Grad. Dip. (Environmental Change Management) Faculty of Science University of the Sunshine Coast January 2009 Submitted in partial fulfilment of the requirements for the degree of Masters in Environmental Change Management Statement of originality I declare that this work does not contain any material which has been submitted by me previously for any degree or diploma to any university, and to the best of my knowledge and belief, it does not contain any material published or written by any other person, except where due reference is made in the text. Prue Pettett January, 2009 2 Abstract Ulva is a common component of marine intertidal flora in Australia with many species frequently observed along the Queensland coastline. Three species of Ulva, U. lactuca, U. intestinalis and U. prolifera were found to naturally occur at the Bribie Island Research Centre (BIRC) in Southeast Queensland. Studies were undertaken to establish the most optimal conditions for growing Ulva in the BIRC laboratory. These tests were conducted in order to condition the algal material prior to the sporulation studies, offering more controlled material to assess treatment effects conclusively, and helping eliminate other potentially confounding environmental factors. Results showed that a stocking density of between 5-20 grams of Ulva per litre along with the addition of the soluble fertiliser Aquasol at a rate of 87 mg/L of seawater was ideal for achieving a desired doubling of growth per week. In the wild the formation of Ulva fragments occurs naturally in the ocean through wave and storm action. This breakage can trigger a survival response mechanism which stimulates the algae to form and release gametes. By chopping the tissue, this process could be artificially simulated in the laboratory and creating a simple and easy way to produce new individuals. Studies performed into inducing sporulation in Ulva through a combination of fragmentation and renewal of medium at BIRC showed that sporulation can be successfully induced in all three species of Ulva through these methods, however it was found to be to a degree that would not meet the demands of commercial production with on average a rate of only 33% achieved. While the current study did not find a method suitable for a commercial application the results presented here contribute to increasing our understanding about Ulva reproduction and set a platform for future work in to cultivating Ulva within Southeast Queensland. 3 Acknowledgements I express my sincere appreciation and gratitude to Dr Paul Palmer, Department of Primary industries, for his valuable guidance and assistance in planning and designing of the experimentation and for his improvements on the manuscript. I also thank Dr Peter Ducan, University of the Sunshine Coast, for his intellectual stimulation and constructive criticism during the writing of this manuscript. I thank Dr David Mayer, Department of Primary industries, for his valuable assistance in the statistical analysis. I also extend a warm thanks to Dr Pia Winberg, Sustainable Seafood Pty. Ltd, for her assistance in identification and to Dr John West, University of Melbourne, and Dr Fred Gurgel, University of Adelaide, for providing answers with their knowledgeable expertise in Ulva reproduction. 4 Table of Contents Abstract..........................................................................................................................................3 Acknowledgements .......................................................................................................................4 List of Figures................................................................................................................................7 List of Tables .................................................................................................................................9 Chapter One: General Introduction ..............................................................................................10 1.1 Taxonomy and biology of Ulva ..........................................................................................10 1.1.1 Introduction................................................................................................................10 1.1.2 Assimilation of the genus Enteromorpha into Ulva ..................................................11 1.1.3 Reproductive biology.................................................................................................13 1.2 Commercial production of Ulva ..................................................................................15 1.2.1 Outline of the current industry...................................................................................15 1.2.2 Commercial uses and bioremediation potential.........................................................16 1.2.3 Seed cultures and future directions ............................................................................17 1.3 Inducing reproduction in Ulva ......................................................................................17 1.3.1 Light...........................................................................................................................17 1.3.2 Temperature ...............................................................................................................18 1.3.3 Changing the culture medium....................................................................................19 1.3.4 Dehydration................................................................................................................20 1.3.5 Thallus fragmentation ...............................................................................................20 1.3.6 Identifying and assessing sporulation ........................................................................21 Chapter Two: Identification of Ulva species. ...............................................................................24 2.1 Introduction.........................................................................................................................24 2.2 Materials and Methods........................................................................................................24 2.3 Results.................................................................................................................................25 2.3.1 U. lactuca...................................................................................................................25 2.3.2 U. intestinalis .............................................................................................................28 2.3.3 U. prolifera ................................................................................................................28 2.4 Discussion ...........................................................................................................................32 2.5 Conclusions.........................................................................................................................34 Chapter Three: Culture studies.....................................................................................................36 3.1 Introduction.........................................................................................................................36 3.2 Materials and Methods........................................................................................................36 3.2.1 Nutrient type and concentration.................................................................................37 3.2.2 Water type..................................................................................................................38 3.2.3 Species selection for fast growth ...............................................................................39 3.3 Results.................................................................................................................................40 3.3.1 Nutrient type and concentration.................................................................................40 3.3.2 Water type..................................................................................................................41 3.3.3 Species selection for fast growth ...............................................................................42 3.4 Discussion .....................................................................................................................44 3.5 Conclusions...................................................................................................................46 5 Chapter Four: Inducing sporulation in Ulva spp..........................................................................47 4.1 Introduction.........................................................................................................................47 4.2 Materials and Methods........................................................................................................48 4.3 Results.................................................................................................................................50 4.3.1 Sporulation.................................................................................................................50 4.3.2 Fouling .......................................................................................................................56 4.4 Discussion ...........................................................................................................................57 4.5 Conclusions.........................................................................................................................61 Chapter Five: Concluding summary .............................................................................................62 References......................................................................................................................................63 Appendix 1: Phytoplankton and other organisms observed in outdoor stock culture and experimental Petri dishes ................................................................................................................69 6 List of Figures Chapter One Fig. 1.1 Schematic view of nuclear orientation and movement during somatic mitosis and meiotic zoospore formation ...................................................................................................12 Fig. 1.2 Typical life cycle of Ulva; A: Male and female gametophyte, B: Biflagellate gamete, C: Sporophyte, D: Quadriflagellate zoospore. 1N: Haploid; 2N: Diploid ...............14 Chapter Two Fig. 2.1 U. lactuca in shallow drain (a), U. lactuca in deep mullet pond (b)........................26 Fig. 2.2 U. lactuca outdoor culture stock (a), U. lactuca thallus (b), Surface view of the thallus cells (c), Transverse section of thallus cells (d) .........................................................27 Fig. 2.3 U. intestinalis outdoor culture stock (a), U. intestinalis thallus (b), Surface view of the thallus cells (c), Transverse section of thallus cells (d) ...............................................30 Fig. 2.4 U. prolifera outdoor culture stock (a), U. prolifera thallus (b), Surface view of the thallus cells (c), Transverse section of thallus cells (d) ...................................................31 Chapter Three Fig. 3.1 Average cumulative weight of U. intestinalis under various nutrient concentrations, mean ± se (n=3), different superscripts represent a significant difference (P<0.05) .................................................................................................................................40 Fig. 3.2 Average cumulative weight of U. prolifera under various nutrient concentrations and water type, mean ± se (n=3), different superscripts represent a significant difference (P<0.05) .................................................................................................................................41 Fig. 3.3 U. prolifera not exposed to nutrients (a) and U. prolifera exposed to nutrients (b) 42 Fig. 3.4 Average cumulative weight of U. intestinalis, U. prolifera and U. lactuca with Aquasol full, mean ± se (n=3), different superscripts represent a significant difference (P<0.05) .................................................................................................................................43 Fig. 3.5 Average amount of grams put on each week by U. intestinalis, U. prolifera and U. lactuca...............................................................................................................................43 Chapter Four Fig. 4.1 Visual template designed in current experiment for ranking sporulation of Ulva in Petri dishes.........................................................................................................................49 7 Fig. 4.2 Thallus in vegetative state (a), Thallus with displaced chloroplasts (b) Thallus forming gametes (c) Thallus with fully formed gametes (d), Thallus after release of gametes (e), gametes swimming in Petri Dish (f)..................................................................51 Fig. 4.3 Time profile of mean sporulation between species, mean ± se (n=12)....................53 Fig. 4.4 Time profile of mean sporulation between treatments, mean ± se (n=36)...............54 Fig. 4.5 Mean sporulation of fragmented and whole thalli in U. prolifera, U. lactuca and U. intestinalis (n=84). ............................................................................................................54 Fig. 4.6 Time profile of mean sporulation between weeks, mean ± se (n=9) .......................55 Fig. 4.7 Water parameter values at the start of each week, mean (n=3)................................56 Fig. 4.8 Culture dish fouling (a) and outdoor culture stock fouling (b) ................................56 8 List of Tables Chapter One Table 1.1 Sporulation scale developed by Lersten and Voth (1960) ....................................22 Table 1.2 Sporulation scale developed by Nordby (1977)....................................................22 Chapter Four Table 4.1 Ordered classification table used to assess sporulation in Ulva species ...............49 Table 4.2 Summary of repeated measures ANOVA with three grouping factors (Species, Treatment, Week) and one trial factor (Time) and their interaction effects on mean degree of sporulation in Ulva ............................................................................................................52 9 Chapter One General Introduction 1.1 Taxonomy and biology of Ulva 1.1.1 Introduction Ulva spp. (Chlorophyta, Ulvaceae) commonly known as sea lettuce, a member of the family Ulvaceae are an early-successional algae quickly taking over new substrate after disturbances (MBARI 2001). Ulva’s opportunistic success is attributed to their morphology which allows rapid nutrient uptake, high capacity to store nutrients, and spontaneous mass reproduction (Santelices and Ugarte 1987; MBARI 2001). These characteristics have allowed Ulva to develop a global distribution with populations documented in Europe (Back 2000), North and South America (Hoffmann and Ugarte 1985; Pringle 1986), Asia (Hiraoka et al. 2003), Pacific Islands (Smith 1947) and Australia (Phillips 1998). Ulva occurs mainly in the littoral zone or near the high sub-littoral zone of the ocean foreshore. It is extremely hardy and capable of existing in a wide range of salinities (2-35%). It often ascends into estuaries, especially when there is an increase in nitrogen from pollution (Fritsch 1956; Niesenbaum 1988). For much or all of its life Ulva is anchored via a disc shape holdfast to a substrate which may include rocks, rope, woodwork or larger algae (Fritsch 1956; Pickett-Heaps 1975; Bold and Wynne 1985). Detachment regularly occurs through natural processes such as storm or wave action or through human disturbances such as ship movements, but interestingly, as 10 long as it can access nutrients and light it can survive and grow whilst floating freely in the water column. 1.1.2 Assimilation of the genus Enteromorpha into Ulva Ulva was one of the first seaweed genera to be named and was recognised originally by Linnaeus in 1753 as a single genus. The species was subsequently separated into two genera, namely Ulva and Enteromorpha by Silva (1952), on the basis of the two significantly different morphologies. The morphology of both Ulva and Enteromorpha characterised by a very thin undulating layer of tissue which is essentially a bilayer of typical ulotrichalean cells containing chloroplasts and a single to multiple pyrenoids (Figure 1.1). The cell axis is generally orientated at right angles to the surface of the thallus with the chloroplasts mostly located on the outer side of the cell, however depending on lighting conditions this can change with the chloroplasts able to move about freely inside the cell (Fritsch 1956; Pickett-Heaps 1975). It is this thin cellular layer that distinguishes the two genuses with Ulva consisting of a distromatic blade (two cells thick) and Enteromorpha a tubular blade that is monostromatic (one cell thick) (Bold and Wynne 1985). However, several lines of evidence, found through molecular investigations (Tan et al. 1999; Hayden et al. 2003; Shimada et al. 2003), and distribution and bacterial studies (Nakanishi et al. 1996), suggest that these generic constructs are artificial and that there is only one valid genus, Ulva. 11 Figure 1.1 Schematic view of nuclear orientation and movement during somatic mitosis and meiotic zoospore formation. (From Braten & Nordby, 1973). 12 Molecular phylogenetic analyses of nuclear ribosomal RNA ITS sequences by Tan et al. (1999) indicated that the two genera Ulva and Enteromorpha should not be separated. Analyses showed that the radical change in gross morphology of the two genera has arisen independently several times through their evolutionary history, suggesting that this morphological flexibility is the result of some form of developmental switch resulting in either blades or tubes. This was supported by further studies conducted on the nuclear ribosomal internal transcribed spacer DNA (ITS nrDNA) (Hayden et al. 2003) and ITS and rbcL in Ulva and Enteromorpha species (Shimada et al. 2003), again confirming that Ulva and Enteromorpha are not separate genera. Bacterial studies by Nakanishi et al. (1996) found that Ulva pertusa lost its typical morphology when cultured in a synthetic media that lacked symbiotic bacteria. It was also found that with the reinfection of certain bacteria that this species can regain its typical foliaceous or tubular morphology. These authors concluded that the direct contact between Ulva and the bacterial strain was necessary for normal morphological growth. Since Ulva is the older genus, there is growing consensus that Enteromorpha should be reduced to the synonymy of Ulva (Nakanishi et al. 1996; Shimada et al. 2003). In the following report therefore only the single genus Ulva will be recognised. 1.1.3 Reproductive biology The reproductive cycle of Ulva is known as an alternation of generations (Dawson 1956). It consists of two phases (see Figure 1.2) a haploid (one set of chromosomes) gametophyte stage which produces biflagellate gametes and a diploid (two sets of chromosomes) sporophyte stage which produces quadriflagellate zoospores (Trainor 1978). These two sequential generations are 13 almost identical in appearance (isomorphic); the only differences being the number of flagella in the motile single celled stages, and the cell sizes with haploid cells distinctly smaller than diploid cells (Nordby 1974; Pickett-Heaps 1975; Trainor 1978). Figure 1.2 Typical life cycle of Ulva; A: Male and female gametophyte, B: Biflagellate gamete, C: Sporophyte, D: Quadriflagellate zoospore. 1N: Haploid; 2N: Diploid (from Beach et al. 1995). In the wild the release of zoosporangia or gametangia collectively known as swarmers has been observed to occur around spring tides; however this may vary between species (Niesenbaun 1988). Studies conducted by Pringle (1986) concluded that U. intestinalis swarmer discharge is not synchronised with the occurrence of spring tides, but rather by the lunar cycle. Reproductive formation due to the lunar cycle was also recorded by Christie and Evans (1962) who observed that swarmer discharge in U. intestinalis peaks 3-4 days prior to the highest tides on each lunar cycle. The lunar cycle was also found to play an important role in the laboratory. U. pseudocurvata under laboratory conditions was found to have 7-day reproductive peaks, however after the introduction of artificial moon light every four weeks the reproductive rhythm became more synchronised with only a monthly release (Luning et al. 2008). 14 Ulva’s life-cycle occurs without the formation of special generative organs. Nevertheless, reproductive material can at times significantly dominate the biomass of these algae e.g. it can be up to 60% of the total biomass in species such as U. lactuca (Nordby and Hoxmark 1972; Niesenbaum 1988). In some cases this mass reproduction can have detrimental consequences for the environment. In shallow coastal eutrophic lagoons or estuaries, primary production from Ulva can become so high that subsequent decomposition processes can lead to oxygen depletion (Sfrisco et al. 1992). This effect is most pronounced in the bottom water or sediment which then causes anoxia and hydrogen sulphide production. These conditions then cause detrimental effects on the thalli themselves with reports that exposure to anoxia and sulphide gradually reduces the growth capacity of U. lactuca (Giordani et al. 1997) 1.2 Commercial production of Ulva 1.2.1 Outline of the current industry The commercial farming of macroalgae (seaweed) has a long history dating back hundreds of years. Currently there are approximately 200 species of macroalgae used worldwide, of which around 10 are intensively cultivated (Zemke-White and Ohno 1999; Wikfors and Ohno 2001). Macroalgae global utilization is on the increase with annual harvests worth around $5-6 billion U.S per year (Wikfors and Ohno 2001; Luning and Pang 2003), making it among the most important cultivated marine organisms. Wet annual harvest weights equate to approximately six million 15 metric tonnes, and 90% of this is produced via culture-based practices in China, Korea, Japan, Philippines, Indonesia, Chile, Taiwan, Vietnam, Russia and Italy (FAO 2001; NAAS 2003). 1.2.2 Commercial uses and bioremediation potential Seaweeds are a staple part of the diet of many people living in countries such as Japan, China and Korea. In particular, species such as Nori (Porphyra spp.), Wakame (Undaria pinnatifida) and Kombu (Laminaria japonica) are used as ingredients in soups, salads and noodles, or are served with meat and/or fish as a vegetable (Radmer 1996). Seaweed extracts are also used in a very wide range of products including soaps, shampoos, cosmetics, toothpaste, textiles, sanitary napkins, air fresheners, paper products, culture media, fungicides, beer, meat, ice-creams, syrups and salad dressings (NAAS 2003). More recently seaweeds are becoming used as a cheap and effective biofilter. Land and oceanbased mariculture creates polluting waste nutrients which consist primarily of either the metabolic waste of the cultured species or broken down uneaten food (Naylor et al. 2000). Macroalgae are well known to be able to partly restore water quality and reduce the environmental impact derived from high nutrient loads (Neori et al. 2004). They have been successfully used as biofilters for numerous aquaculture species including abalone (Neori et al. 2000), shrimp (Jones et al. 2002), fish (Neori et al. 1996) and sea cucumbers (Wang et al. 2007). 16 1.2.3 Seed cultures and future directions Traditionally, macroalgae species for mariculture have been obtained through what is known as “natural seeding”. This method involves placing nets, ropes and other spore settlement devices into wild populations during early autumn and spring when the algae are naturally sporulating. However this is becoming less feasible as wild stocks in many countries have been depleted by overharvesting and excessive pollution (Niesenbaum 1988; NAAS 2003). Other reasons resulting in a switch from natural seeding to artificial reproductive induction include minimising variability in production levels by reducing genetic diversity and reducing seasonal effects by allowing reproduction to occur at almost any time of the year when supplied with sufficient parental biomass (Niesenbaum 1988; Ohno 1993; Dan 2002). 1.3 Inducing reproduction in Ulva Vegetative cells can transform directly into zoosporangia or gametangia at any time, and this reportedly can be stimulated through a number of environmental shock treatments including changes in photoperiod, temperature, salinity, nutrients, pH, drying and through fragmentation (Pickett-Heaps 1975; Hiraoka and Enotomo 1998). 1.3.1 Light As the release of reproductive cells is dependent on photosynthesis, light is an important factor in the spawning of Ulva, with a positive correlation between availability of light in the water column and spore biomass. It is believed that the increase in light along with other environmental factors is responsible for the induction of reproductive material in spring and summer (Sousa et al. 2007). 17 This idea was also previously noted by Smith (1947) who believed that sporulation in Ulva species was related to periodic increases of light exposure. The light requirements for maximal spore release in U. pertusa were found by Han et al. (2008) to be >30 μmol/m2. However, Dan et al. (2002) found that Ulva could still reproduce reasonably well at lower light levels, in the order of 16 μmol/m2. The minimum light requirements for reproduction have been found to be much lower at between 5 and 10 μmol/m2 (Dan et al. 2002; Han et al. 2003). 1.3.2 Temperature Ulva is extremely tolerant to temperature changes with freezing experiments conducted by Kamermans et al. (1998) demonstrating that the algae are able to survive at -5ºC for 2 weeks when kept in the dark. This refers only to plant tissue, since sexual reproduction at this temperature is negligible. Maximal sporulation percentages occurred between 15 and 20°C in U. pertusa (Han et al. 2005). There were significant declines at or below 10°C and at or above 25°C and that at 5°C no sporulation occurred. Earlier work showed that the optimum temperature for sporulation of U. mutabilis is 21-22°C (Nordby 1977). However, Nordby also concluded that temperature for sporulation is by no means absolute if given enough time to sporulate. When U. mutabilis fragments were incubated for 10 days, high degrees of sporulation (>50%) could occur in temperatures as high as 28-29°C and as low as 15°C. 18 Rapid changes in temperature can also induce reproduction in Ulva. Washing U. lactuca in filtered seawater at 2°C and then returning it to a similar medium at 22°C caused all of the algal material to become reproductively active within 18 hours (Niesenbaum 1988). 1.3.3 Changing the culture medium Renewal of the suspension medium has also been shown to induce generative division in vegetative cells. This is reported to be well accepted and common practice in laboratories wanting to induce reproduction in Ulva (Nilsen and Nordby 1975). Increasing proportions of freshly added medium, the amount of sporulation also increased (Thaidens and Zeuthen 1967). This is believed to be caused by the associated changes in salinity and pH that stimulate the formation and release of zooids. Salinity can influence spore release by affecting the turgor pressure and diameter of the exit pore of sporangia (Han et al. 2008). Spore germination and growth is adversely affected by low salinities; growth is significantly reduced at salinities below 5 ppt (Sousa et al. 2007). Growth was also found to be highest at levels above 20 ppt, and with 35 ppt the most effective. Other species have also shown a tendency for optimal spore release rates at oceanic salinities (35 ppt). For example, Han et al. (2008) found that maximal spore release for U. pertusa occurred between 25 and 35 ppt. The pH of the suspension medium has also been shown to affect sporulation in at least two Ulva species. The optimum pH for inducing sporulation in U. mutabilis is thought to be 8.0-8.5 (Nordby 1977), and the optimum pH for spore release in U. pertusa is reportedly between 7 and 9 (Han et al. 2008). 19 1.3.4 Dehydration Since Ulva occurs naturally in the tidal zones of oceans, it is periodically subjected to varying levels of dehydration. Sporulation could be induced by dehydration in U. lobata, U. taeniata, U. linza, U. angusta and U. stenophylla (Smith 1947). The procedure involved removing thalli from the water for an hour and then re-immersing them. When treated in this manner most of the blades reportedly released swarmers within 5-10 minutes. However, it is probable that in this case the first phase of reproduction had already been completed and that the algae just needed another stimulus to complete the second phase of reproduction. Corradi et al. (2006) induced gametogensis in Ulva by dehydrating the thallus on a filter paper at 20 ± 1°C and 25% humidity for 10, 20, 30 and 40 minutes in a climate-controlled chamber. In contrast with Smith (1947) thalli subjected to dehydration took three days to produce gametes; presumably in this case it was not already reproductively mature. 1.3.5 Thallus fragmentation Fragmentation has been found to be a highly successful form of treatment to induce sporulation and has been used to stimulate the release of zooids in Ulva for many years (Nordby 1974; Dan et al. 2002). Fragmentation can greatly improve sporulation rates with studies finding that tearing of the thallus can increase sporulation from 15.8 to 80% (Nordby 1977). The cutting of the thalli has also been found to improve synchronisation. Research by Nordby and Hoxmark (1972) reported a high degree of synchronous zooid formation by cutting the thallus of U. mutabilis with razor blades into small fragments of around 0.4 mm x natural width. However this contradicted previous findings by Lersten and Voth (1960) who found no significant differences in zooid discharge between fragmented and non fragment thalli. However, Lersten and Voth used comparatively 20 larger thalli fragments (4 mm) than used in other studies, and this may explain their differing results. Studies by Hiraoka and Enomoto (1998) into fragment size found that there were significant differences in the degree of sporulation between fragment sizes. They concluded that zooid formation increases as fragment size decreases. Nordby (1977) found that fragments that are too small should be avoided since the edge cells tend to sporulate later than the inner cells. Various methods and instruments have been used to experimentally fragment the thalli including tearing the thalli with needles (Nordby 1977), with a razor-blade blender (Nordby and Homark 1972), with a leather craft punch (Dan et al. 2002; Hiraoka 2003; Hiraoka and Oka 2008) or a double-bladed ophthalmic knife (Lersten and Voth 1960), as well as razor blades (Verlaque et al. 2002) and herb choppers (Stratmann et al. 1996). Using a leather craft punch is now becoming a more favoured experimental method since the circular shape creates more surface area exposed to fragmentation, however there are no documented comparisons of the effectiveness of various instruments, and there appears to be no commercially applicable method that is suitable for large volume treatments. 1.3.6 Identifying and assessing sporulation Reproductive maturation usually occurs within two to three days of applied stimuli, and often between the hours of 11:00 pm and 4:00 am. Swarmer discharge occurs the same day, generally a few minutes after sunrise (Carter 1926; Hiraoka and Oka 2008). The sex of biflagellate gametes can be distinguished from each other by their colour; female gametes appear as a dark green mass while male gametes appear more yellow green in colour due to their prominent eyespot. 21 On a broad scale, sporulation can be identified in Ulva thalli through a change in colouration. During the vegetative state the thallus is a dark or slightly yellow green. However, during reproductive maturation, the thallus turns to a dark olive to orange-brown (Hiraoka and Enomoto 1998; Han and Choi 2005). When the zooids are released the thallus becomes colourless in contrast to green non-discharged sections, and this presents an opportunity to rate levels of sporulation by eye in a qualitative fashion. Various sporulation scales have been developed to assist in gauging the level of sporulation through assessing the degree of change in colour of the thallus. Lersten and Voth (1960) developed the following simple scale based on how much green colour remained in the thallus (Table 1.1): Table 1.1 Sporulation Scale developed by Lersten and Voth (1960). Discharge level No discharge Discharge less than one-third of the thallus Discharge from one-third of the thallus Discharge from more than two-thirds of the thallus Value 0 1 2 3 Nordby (1977) taking a similar approach however developed five categories which were weighted for sporulation as follows (Table 1.2): Table 1.2 Sporulation Scale developed by Nordby (1977). Discharge level 0% discharged >10% discharged 10-50% discharged 50-90% discharged 90-100% discharged Value 0 0.5 3 7 10 22 Sporulation can also be measured through quantitative measurements including measuring the density of the zooid with a haemocytometer and by measuring the chlorophyll a concentration of the suspending medium (Niesenbaum 1988; Hiraoka and Oka 2008). The objectives of the following study were to; identify all Ulva species naturally occurring at BIRC (i), establish the most optimum conditions for growing Ulva in the laboratory (ii), test if Ulva can be successfully induced into sporulation through a combination of fragmentation and renewal of medium and if so quantify the degree of sporulation and assess if it is suitable for a commercial application. 23 Chapter Two Identification of Ulva species 2.1 Introduction Ulva is a common component of marine intertidal flora in Australia with many species frequently observed along the Queensland coastline (Cribb 1956). An extensive literature search has found that there is good documentation of species diversity within Queensland with the following species of Ulva recorded to occur; U. ahlneriana, U. clathrata, U. compressa, U. fasciata, U. flexuosa, U. gunniana, U. intestinalis, U. lactuca, U. linza, U. paradox, U. procera, U. prolifera, U. ralfsii, U. reticulata and U. rigida, however there is very limited information available about the exact locations and seasonal abundance of this genus within Queensland (Cribb 1956: Cribb 1996; Phillips 1997; Davie 1998; Phillips 2007). Natural populations of Ulva were found to regularly occur in various places at the Bribie Island Research Centre (BIRC) in Southeast Queensland, however their taxonomy was uncertain. The following study sought to identify all Ulva species occurring at BIRC and document their morphological characteristics and habitat for future reference. This was seen as a necessary starting point for using this source of Ulva species in sporulation induction trials. 2.2 Materials and Methods Thalli of Ulva were collected from outside drains and ponds at BIRC in April 2008. The thalli were collected and photographed immediately with the use of a digital camera and light microscope (Nikon Microphot-FXA), at various levels from broad scale views to microscopic. 24 Transverse sections were taken by holding the thallus down with a coverslip and finely slicing the thalli with a razor blade; this was later photographed with the use of a light microscope. Cellular and thallus measurements were taken with the use of a ruler and micrometer. These photographic records, cellular measurements, and thalli measurements along with further live samples were used to sequentially identify the species present at that time. Identification was conducted in collaboration with Dr Pia Winberg, director of Sustainable Seafood Pty. Ltd who had prior experience with Ulva identifications. Generally, species are primarily distinguished based on anatomical grounds using the dimensions, length and breath ratios and lateral profiles observed in transverse sections, including blade morphology such as branching (Phillips 1988). Identification was assisted with use of the following guides: Cribb (1956), Phillips (1988), Cribb (1996) and Kraft (2007). Habitat descriptions were noted and abundance was visually observed and recorded over a seven month period (April to October). 2.3 Results The study of the BIRC facility established that three species of Ulva are commonly present. These species were identified as U. intestinalis, U. prolifera and U. lactuca. Their habitat and biology descriptions were made as follows: 2.3.1 U. lactuca U. lactuca was found growing both in shallow drains with less than 2 cms of water and in 1-2 m deep ponds. U. lactuca was noted to be growing under the following conditions: full sun; slow to still water movement in ponds; fast and strong water movement in drains; natural seawater salinity with higher than natural nutrients levels due to nutrient supply from aquaculture ponds. The 25 morphological structure was seen to change with different water depths, with a more crumpled and compact form in shallow areas and a broader more flat form in deeper waters (Figures 2.1 and 2.2). In general it can be described as a thin flat green alga with broad and crumpled thalli 18 cms or more in length and up to 30 cm wide. There were significant variations in the size of this species detected, with the largest specimen observed being 77 cms long and 33 cms wide. The alga was found both growing attached via a small disc-shaped holdfast and also unattached floating on the surface. The thallus is generally translucent and green to dark green in colour, however yellowing occurs during times when there are only limited nutrients. Thalli were also found to bleach white when exposed to air for extended periods of time. The thallus in U. lactuca is distromatic (two cells thick) with each of the cells containing one to three pyrenoids. Cells are irregularly arranged, cell diameter in surface view is 10-15 μm, and thallus thickness is 80 μm. Abundance of this species was found to be at highest during the months of August, September and October. a b Fig. 2.1 U. lactuca in shallow drain (a), U. lactuca in deep mullet pond (b). 26 a b 15 cms c d 10 µm 10 µm Fig. 2.2 U. lactuca outdoor culture stock (a), U. lactuca thallus (b), Surface view of the thallus cells (c), Transverse section of thallus cells (d). 27 2.3.2 U. intestinalis U. intestinalis was found growing both attached (via a disc-like holdfast) and unattached in shallow drains (>2 cms) and deep ponds (1-2 m) in troughs at BIRC (Figure 2.3). It was noted to be growing in the following conditions: full sun; slow to still water movement; natural seawater salinity with high nutrients levels from aquaculture ponds. It was observed to form thick mats (up to 5 cm thick) which floated on the water surface when grown in deep ponds. U. intestinalis can be described as having a thin ruffled unbranched fronds 1 cm wide and up to 20 cms or more in length. The shape is variable with some widening in the mid region. Thalli are bright green in colour with yellowing occurring when exposed to a lack of nutrients. The thallus is monostromatic with one to four pyreniods per cells. Cell diameter in surface view is 10 μm, thallus thickness is 30 μm. U. intestinalis was found to be highest in abundance at BIRC during the months of April, May and June. 2.3.1 U. prolifera U. prolifera has thin hollow tubular monostromatic thalli which are bright green to dark green in colour (Figure 2.4). Again, yellowing is observed to occur when there is a lack of nutrients. It was found both attached via a disc-like holdfast and unattached in shallow drains (>2 cms) and deep ponds (1-2 m). The thalli were found exposed to full sun, natural seawater salinity, with nutrient supply from aquaculture ponds and slow bubbling water movement. 28 The thallus is 1 mm wide and 10-15 cms in length, cell diameter in surface view is 10 μm, thallus thickness is 30 μm. Abundance of U. prolifera was found to be highest at BIRC during the months of June, July, August, September and October. 29 a b 5cms c d 10µm 10µm Fig. 2.3 U. intestinalis outdoor culture stock (a), U. intestinalis thallus (b), Surface view of the thallus cells (c), Transverse section of thallus cells (d). 30 a b c d 10µm Fig. 2.4 U. prolifera outdoor culture stock (a), U. prolifera thallus (b),Surface view of the thallus cells (c), Transverse section of thallus cells (d). 31 2.4 Discussion The three species identified at BIRC in this study, U. lactuca, U. prolifera and U. intestinalis, have been reported from this part of Queensland by several previous authors (eg: Cribb 1956: Cribb 1996; Phillips 1997; Phillips 2007). Hence, there is reasonable confidence that the species identifications in this study are correct. Nevertheless, it should be recognised that some uncertainty in this regard still exists. Ulva presents extreme difficulties in taxonomy due to its wide intraspecific variations in morphologies. Morphological differences between species are notably small and difficult to detect (Bliding 1963). While it was easy to discriminate between the species classified in this study as U. lactuca, U. intestinalis and U. prolifera, because they each have very distinct growth forms, it is generally not so easy to distinguish these species from other very similar species within this genus. Species such as U. lactuca have been noted to resemble other species, such as U. linza, and it seems likely that within Australia these species have been frequently confused (Phillips and Clayton 1983). Another set of species which have been misidentified are U. intestinalis and U. compressa. These two species are morphologically so similar that they are almost indistinguishable. One morphological characteristic used to separate these two species is the degree of branching; U. intestinalis is generally unbranched but U. compressa takes on a branched morphology. However under different environmental conditions this branching tendency can vary. In brackish water conditions U. intestinalis can become branched and U. compressa can revert to an unbranched form, further complicating attempts at morphological discrimination (Tan et al. 1999). This ability for Ulva to switch morphological characteristics under different environmental conditions makes Ulva taxonomy difficult. 32 The present study recognised the need to document habitat descriptions when identifying the species. In the current study significant morphological changes were observed in U. lactuca under different environmental conditions. When grown in a deep still pond the thalli were flat and smooth however in shallow high water flow drains the thalli were compact and ruffled (see Fig 2.1). This is believed to have occurred due to the changes in water movement. Similar gross changes in size and morphology in Ulva have been observed in other studies. Mshigeni and Kajumulo (1979) found U. fasciata to change morphology when exposed to different water conditions with thalli becoming smooth and long when placed in calm-water and more compact and dwarf like when placed in wave-exposed water. The identification techniques employed in the current study are considered to be a scientifically acceptable form of identification (Phillips 1988); however more recently, nuclear ribosomal DNA internal transcribed spacer (ITS) sequences have been used for the identification of Ulva species (Dion et al. 1998). These rDNA data have been accumulated and can be utilised for comparison among difficult to identify Ulva species, and of course they are much more accurate for identification (Blomster et al. 1998; Shimada 2003). This was however not feasible in the current project due to limited resources. Ulva is known as a pseudo-perennial with most of the thalli dying back during warmer months however the holdfast portions are perennial and new thalli are then regenerated through this basal material during cooler times of the year (White and Keleshian 1994). An extensive literature search found very little documentation of seasonal changes with most studies not going any further than stating that Ulva abundance is highest during the cooler months (eg: Cribb 1996). Outdoor Ulva assemblages at BIRC were seen to fluctuate significantly between months. This is yet to be fully investigated but could possibly be due to a number of factors including changes in nutrient input, growth, salinity, temperature, light, grazing pressure from invertebrates, sporulation and 33 decomposition which have all being previously found to change Ulva biomass (Sfriso & Marcomini 1996; Balducci et al. 2001). This fluctuation in Ulva assemblages was not viewed by scientists at BIRC as a significant problem for its use as a bioremediation agent, since one useful species seems to give way to another in a timely and uninterrupted fashion. In other words, when one species of Ulva sp. died off another increased in occurrence, filling the niche space and utilising the available light and nutrient resources. Therefore, its nutrient stripping abilities were maintained as long as a reasonable biomass of at least one form was maintained. The abundance of Ulva at BIRC may not be representative of wild populations under unpolluted natural conditions, because Ulva in certain localities at the research station is under more favourable conditions having higher nutrient supplies, stable water levels with no tidal effects or storm movement, and metered control over herbivorous grazers. In order to better understand wild populations of Ulva in Southeast Queensland further studies would need to be broadly undertaken in its many suited habitats along the Queensland coastline. 2.5 Conclusions Three species of Ulva were found to occur naturally in drains and ponds at BIRC. The species were identified as U. lactuca, U. intestinalis and U. prolifera. Whilst there is reasonable confidence that the species were identified correctly, some uncertainty still remains due to the extreme difficulties in Ulva taxonomy. Molecular analysis would need to be undertaken in order to obtain complete certainty however this was not feasible in the current study. Seasonal variations in outdoor Ulva populations were observed to occur. The reason behind the increases and decreases in biomass is yet to be investigated, however some possibilities include changes in growth, nutrients, salinity, temperature, light, grazing pressure from invertebrates, sporulation and decomposition. Further studies need to be undertaken over several full years in order to better 34 understand the population dynamics of U. prolifera, U. lactuca and U. intestinalis at BIRC. Observations of Ulva abundance at BIRC may not reflect wild populations because Ulva in the outdoor water system at BIRC can at times be presented with very favourable growth conditions. Further studies would need to be undertaken to better describe the patterns of growth and to assess the true abundance of Ulva in the wild within Southeast Queensland. 35 Chapter Three Culture Studies 3.1 Introduction Ulva is a hardy alga that is highly tolerant of variable salinity, temperature and water quality. It grows rapidly in nutrient-rich habitats and is often used as a bioindicator of organic and inorganic pollution in waterways (Fletcher 1996; Leal et al. 1997). Ulva is so successful at absorbing nutrients that it can be integrated into land-based aquaculture practices and used as a biofilter partly restoring the water quality and reducing the environmental impact derived from the high nutrient loads (Neori 1996; Neori et al. 1996; Neori et al. 2004). Ulva is currently growing successfully in outdoor troughs and drains at BIRC, however in order to undertake sporulation studies the alga must first be successfully grown in the laboratory. The following chapter aimed to establish the optimal conditions for growing Ulva in the BIRC laboratory. This would allow it to be conditioned prior to sporulation studies, offer more controlled material to assess treatment effects conclusively, and help eliminate other potentially confounding environmental factors. 3.2 Materials and Methods Three different experiments were conducted as a part of optimising culturing conditions in the laboratory. They focussed on: (1) optimising available nutrient types and concentrations; (2) investigating the effect of different source waters; and (3) assessing comparative growth rates of the various species to identify the fastest grower under the experimental conditions. 36 3.2.1 Nutrient type and concentration U. intestinalis was gathered from outdoor stock cultures at BIRC and washed in fresh water three times to kill potential grazers (eg: amphipods). These wild gatherings were divided into 10 g lots (wet weight). Each of these 10 g lots was then placed into a 3 L clear culturing bowl containing 2 L of filtered (1 µm) seawater and one of the following five treatments. Nutrient concentrations were based on a desired doubling of growth per week which would require an assumed 40 mg of nitrogen per week: • No added nutrients (seawater control) • Full strength Aquasol soluble fertiliser - 87 mg per litre • Half strength Aquasol soluble fertiliser - 43.5 mg per litre • Full strength Nanno mass culture mix - 199 mg per litre • Half strength Nanno mass culture mix - 99.5 mg per litre The Aquasol fertiliser used is a commercially available product which provides a balanced nutrient mix containing the full range of trace elements necessary for plant growth. It is easily dissolved in seawater and is routinely used at BIRC to grow the marine microalgae Isochrysis galbana. The Nanno mass culture mix is a mixture of agricultural grade fertilisers that are used to grow mass cultures of the marine microalgae Nannochloropsis oculata. It consists of ammonium sulphate, superphosphate and urea in the w/w ratio of 15.3: 1.4: 1.0 (Palmer et al. 2007). Five replicates of each of these treatments were made giving a total of 25 experimental units. Each bowl was randomly placed in a light cupboard using a random table of numbers. Treatments were cultured under laboratory conditions at ambient temperature (approximately 20˚C) and exposed to 37 a white light at 10,000 lux under a 12 hour light: 12 hour dark regime for two weeks. Weight measurements were recorded at the same time every week by shaking the algae dry in a fish net and then weighing on an electronic scale (0.01 g accuracy). 3.2.2 Water type U. prolifera material was collected from outside stock cultures at BIRC and washed in fresh water three times. Drainage water was collected weekly from a cultured-marine-worm sand bed and passed through a filter to 1 µm prior to use. Seawater used in the trial was also filtered to 1 µm. 10 grams of U. prolifera was placed into each 3 L clear bowl with 2 L of either water type and one of the following treatments was applied: Seawater - full Aquasol - 87 mg per litre Seawater - no nutrients Worm bed water - full Aquasol - 87 mg per litre Worm bed water - no nutrients Five replicates were made for each of the treatments making a total of 20 experimental units. Each bowl was placed in a light cupboard (10,000 lux) on a 12hr light: 12 hr dark cycle at ambient temperature (approx 20˚C) for three weeks. At the same time every week wet weight measurements were taken by shaking the algae dry in a net and then weighing on electronic scales. 38 3.2.3 Species selection for fast growth U. prolifera, U. lactuca and U. intestinalis were collected from outdoor stock cultures at BIRC. The algal material was then washed in fresh water 3 times and divided into nine 10 g lots. Each of the 10 g lots were then placed in a 3 L clear bowl with 2 L of nutrient enriched (Aquasol full strength; 87 mg/L) seawater. The bowls were randomly placed onto a wire rack in a light cupboard on a 12 hr light: 12 hr dark cycle at ambient temperature (approx 20˚C) for two weeks. Once a week the wet weight of algae (shaken dry as above) was recorded for each of the experimental units. Data Analysis In the nutrient type and concentration experiment a one-way ANOVA and Tukey’s HSD post hoc test were performed with the use of the statistical package SPSS (14.0) to assess if there were significant differences between the five nutrient types. In the water type experiment a one-way ANOVA was performed to test for the effects of water type on growth of U. prolifera (P=0.05). If the ANOVA was significant, a Tukey’s HSD test was applied to test for significant differences within the factor levels (P=0.05). For the species selection experiment a one-way ANOVA and Tukey’s post-hoc test was be performed to assess the significance of differences between the growth of U. intestinalis, U. lactuca and U. prolifera. 39 3.3 Results 3.3.1 Nutrient type and concentration Aquasol full concentration experienced the highest quantity of growth throughout the experiment with a gain of 22.65 grams within two weeks (Figure 3.1). This fertiliser treatment produced significantly higher weight gains than all other treatments except the Aquasol half treatment (which gained 18.47 grams in two weeks). Nanno full performed the worst in terms of growth with only 5.80 grams gained in two weeks. A one-way ANOVA determined that there was a significant difference in growth between nutrient types and nutrient concentrations (P<0.05). Post-hoc analysis (Tukey’s test) determined that the difference is located between the control and Aquasol full (P<0.001), Aquasol full and both Nanno full (P<0.001) and Nanno half (P<0.05) and finally between Aquasol half and Nanno half (P<0.05) (Fig 3.1). 40 c Weight (grams) 35 b,c 30 a,b a 25 20 a Nano Full Control Nano Half 15 Aquasol Half 10 Aquasol Full 5 0 Initital Week 1 Week 2 Time Fig. 3.1 Average cumulative weight of U. intestinalis under various nutrient concentrations, mean ± se (n=3), different superscripts represent a significant difference (P<0.05). 40 3.3.2 Water type Treatments applied in the water type experiment were shown to significantly effect the results (P<0.001) (Figure 3.2). Post-hoc analysis (Tukey’s test) determined that the addition of Aquasol significantly increased algal weight gains however there were no statistically significant differences observed between water types (P>0.05). b 50 b 45 Weight (grams) 40 35 a 30 Wormbed Control a Seawater Control 25 Wormbed Full Aquasol 20 Seawater Full Aquasol 15 10 5 0 Intitial Initial Week Week 1 1 Week Week 2 2 Week Week 3 3 Time Time Fig. 3.2 Average cumulative weight of U. prolifera under various nutrient concentrations and water type, mean ± se (n=3), different superscripts represent a significant difference (P<0.05) 41 Through visual observation it was found that nutrient availability changed the colour of the thallus. Ulva thalli exposed to no-nutrients treatments became pale green to yellow in colour whereas Ulva thalli treated with a strong nutrient concentration turned dark green (Figure 3.3). a b Fig. 3.3 U. prolifera not exposed to nutrients (a) and U. prolifera exposed to nutrients (b). 3.3.3 Species selection for fast growth All three species experienced a high degree of growth during the two weeks of experimentation. U. lactuca demonstrated the highest weight gains with an increase in wet weight of 71.7%. It was closely matched by U. prolifera with a 70.7% increase in wet weight. The least amount of growth was seen in U. intestinalis with a final average weight of 12.84 grams or an increase of 28.4%. A one way ANOVA performed showed that there was a significant difference in growth between species (P<0.05) (Figure 3.4). 42 Post hoc analysis (Tukey’s test) established that the weight gains of U. prolifera and U. lactuca were not significantly different (P>0.05). However there was a significant difference between U. intestinalis and U. prolifera (P<0.05) and between U. intestinalis and U. lactuca (P<0.05). b 20 Weight (grams) 18 16 b a 14 U. intestinalis 12 10 U. prolifera 8 U. lactuca 6 4 2 0 Initital Week 1 Week 2 Time Fig. 3.4 Average cumulative wet weights of U. intestinalis, U. prolifera and U. lactuca with Aquasol full, mean ± se (n=3). Different superscripts represent a significant difference (P<0.05). Growth patterns of U. intestinalis were observed to be exponential and in the other two species it appeared to be more linear (Figure 3.5). 6 Weight (grams) 5 4 U. lactuca U. prolifera 3 U. intestinalis 2 1 0 Initial Week 1 Week 2 Time Fig. 3.5 Mean weekly weight gains for U. intestinalis, U. prolifera and U. lactuca. (n=3) 43 Discussion The rapid growth of all three algal species seen in the current experiment is due to Ulva’s highly efficient morphology. Its simple double-celled sheet-like thalli or single-celled hollow thalli enables the species to respond rapidly to nutrient inputs with high rates of nutrient uptake and growth. Based on the growth comparisons that were conducted in this work, either U. lactuca or U. prolifera would provide maximum growth properties for optimum nutrient assimilation capacities in a bioremediation system. Opportunistic seaweeds such as Ulva have a high demand for nitrogen thus without sufficient nitrogen concentrations growth is limited. Seaweeds can utilise nitrogen in several forms including ammonia, nitrite, nitrate and as dissolved organic nitrogen, but ammonia is the most efficient form for plant uptake (Neori 1996). Mariculture wastewater often high in nitrogen content, so the culture of seaweed such as Ulva sp. in these waste streams is a logical step towards improved productivity and reduced environmental harm from such activities. The importance of nitrogen for Ulva growth is well demonstrated in the current experiment, both through the resultant colouration and weight gains in the water type experiment. The nutrient concentration Aquasol full strength (87 mg per litre) used in the current experiment appeared to be ideal with extremely high growth rates recorded. This fertiliser has a range of balanced trace elements, compared with the Nanno mix which is mainly nitrogen and phosphorus based. This more balanced nutrient availability may explain the present results. Without sufficient nutrients Ulva growth was observed to be significantly less and a visual change in colouration was observed with a yellowing of the thallus. Whilst nitrogen is one of the nutrients which has primary importance for plant growth, phosphorus is also a major limiting nutrient in algal blooms. Dissolved forms of both of these major nutrients 44 are generally available in plentiful supply in the drainage water from sand worm beds used to filter mariculture waste water (Palmer 2008). This is due to the bacterial breakdown of particulate organic matter that is continuously deposited in the beds. Several other factors add to the presumption that this worm bed drainage water will be very suitable for Ulva growth. These include the reduction of microalgal densities it affords which increase light penetration into the water column, and the removal of grazing herbivores like amphipods which can prevent harvestable quantities of Ulva from developing in culture vessels. Since researchers at BIRC are therefore interested in developing systems to grow Ulva sp. in this worm bed drainage water, the present study sought to compare it with seawater. The results of this comparison suggest that equal growth can be achieved in either medium as long as sufficient nutrients are present. Although we had expected faster growth in the worm bed water, due to the background availability of dissolved nutrients, other factors may have played a part in the results and will need further investigation. These include the presence of other chemicals (eg: sulphur) that may impede algal growth, or possibly competition with bacteria for ammonia. Because amphipods were not present in the seawater growth medium, and were removed from seaweed seed stock as it entered the laboratory with freshwater washing, this factor that is important in outdoor situations played no part in these experiments. Light is also well recognised as an important factor in Ulva growth. If Ulva is stocked at very high densities its growth is impeded through self shading (Bartoli et al. 2005). In this regard, Jiménez del Río et al. (1996) have recommended that Ulva should not be stocked at densities greater that 1.5 grams of wet weight per litre. Yet in the current experiment the stocking density was 5-20 grams per litre and growth was still maintained at a high level. This is likely because the Ulva in this study was grown in the laboratory was under fairly intense lighting (10,000 lux), and because 45 the culture vessels were clear bowls illuminated unilaterally by a bank of fluorescent tubes. This allowed Ulva throughout the entire culture to receive a high level of light. 3.5 Conclusions Methods were developed which could produce rapid growth of Ulva in controlled laboratory cultures; rapid growth was demonstrated under high nutrient concentrations and Aquasol full strength was found to be an ideal fertiliser for optimal Ulva growth in the BIRC laboratory. Although previous experiments had found that Ulva stocking density should not exceed 1.5 grams per L in the current experiment it was established that much higher stocking rates could be used because the unilateral banks of lights enabled Ulva throughout the culture vessels to receive sufficient quantities of light. While it is likely that drainage water from a worm bed will provide more favourable outdoor conditions for Ulva growth than untreated mariculture effluent, due to greater light availability, more dissolved nutrients, and a lack of herbivorous grazers, no significant growth differences were detected between seawater and worm bed water in the present work. Future studies should be undertaken to explain why worm bed water was not seen to improve Ulva growth under laboratory conditions. 46 Chapter Four Sporulation Studies 4.1 Introduction In the wild Ulva reproduction has an apparent correlation with tides and phases of the moon (Smith 1947). In the laboratory, various studies have shown that Ulva can be successfully induced into reproduction through a number of stressors including: raising or lowering the temperature; increasing or decreasing light intensity; transferring to darkness; transferring from still to moving water; increasing or lowering the concentration of nutrient compounds in the medium; and renewing the medium. The last method combined with tearing of the thallus has been used as the principal method for inducing reproduction in Ulva sp. in the laboratory in numerous previous experiments (Nordby 1977). While extensive testing has been conducted on induced sporulation in countries such as USA (Smith 1947; Lersten and Voth 1960; Niesenbaum 1988), Japan (Hiraoka and Oka 1998; Dan et al. 2002; Hiraoka and Enomoto 2008) China (Lin et al. 2008), Norway (Braten and Nordby 1973; Nordby 1974; Nordby 1977) England (McArthur and Moss 1979) and Denmark (Thiadens and Zeuthen 1967) currently there are no published studies on this subject from southeast Queensland. Furthermore, many of the methods that have been used are not very applicable to a commercial situation where large scale treatment will be necessary. The following experiment therefore aimed to assess these aspects of induced sporulation of Ulva in southeast Queensland. The methods tested were a combination of renewal of medium and fragmentation, and considerations were given to the suitability’s of these methods for a commercial application. 47 4.2 Materials and Methods Well-developed thalli of U. prolifera, U. lactuca and U. intestinalis were collected from outdoor stock cultures at BIRC on August 18, 2008. After collection, the thalli were washed three times in fresh water to remove any potential grazers and then placed under laboratory conditions for two weeks in the conditions previously determined for optimal vegetative growth. After the two weeks of conditioning the algal material was assessed visually for reproduction and any algal material that was currently in a reproductive state was excluded from the experiment. By use of a razor blade the selected thalli were neatly and quickly cut into segments 5 x 5 mm for U. lactuca, and 5 mm x natural widths (1 mm - 10 mm) for U. prolifera and U. intestinalis. Segmented and whole thalli were then transferred into clean Petri dishes containing 30 mls of fresh 1 µm filtered seawater enriched with Aquasol (87 mg/L) using 1 g of Ulva per dish. Each Petri dish was assigned a number from a table of random numbers and randomly placed onto a wire bench situated inside a laboratory. The Petri dishes were housed at ambient temperature (approximately 20°C) and illuminated unilaterally by banks of white fluorescent tubes attached to the wall at 10,000 lux for 11 days. Photoperiod was run on a 12 h dark: 12 light cycle by an automatic time clock. Results of the experiment were judged by the number of gametes released from the thalli. As sporulation tends to be expressed in the form of sharply divided areas of sporulating and nonsporulating cells, rather than intermingling of the two cell types, there is a distinctive colour difference between green non-discharged areas of the thallus and colourless discharged areas enabling each Petri dish to be visually graded (Nordby 1974). Gamete discharge was measured using a 0 to 3 scale previously employed by Lersten and Voth (1960) (Table 4.1 and Figure 4.1). 48 Table 4.1 Ordered classification table used to assess sporulation in Ulva species (after Lersten and Voth, 1960). Sporulation scale No discharge Discharge less than 1/3 of thallus cells Discharge 1/3 of thallus cells Discharge more than 2/3 of thallus cells 0 1 2 3 0 = No discharge 1 = Discharge less than 1/3 2 = Discharge 1/3 3 = Discharge more than 2/3 Fig. 4.1 Visual template designed in current experiment for ranking sporulation of Ulva in Petri dishes. Each Petri dish was visually inspected at 12:00 noon at 24, 48, 72, 96, 168, 216 and 264 hrs after the start of the experiment. The entire experiment was then repeated a further three times to see if the level of sporulation varied throughout the month. Water parameters including temperature, 49 dissolved oxygen, salinity and pH were measured in three randomly selected experimental units at the start of each new experiment. Observations of fouling were noted and photographs were taken of the phytoplankton species which proliferated. The phytoplankton was then identified with the use of the following guides: Foged (1987), Zheng Zhong (1989), Round et al. (1990), Hasle and Syvertsen (1996) and Stafford (1999). Data Analysis A repeated measures ANOVA was conducted with the use of the statistical software GenStat (11th edition) to analyse sporulation data (Rowell and 1976). A 5% level of statistical significance was used, when data sets significantly violated the requirements for a repeated-measures design ANOVA, the Greenhouse Geisser Epsilon correction was used to calculate a more conservative P value for each F ratio (Greenhouse and Geisser 1959). The statistical package SPSS (14.0) was used to determine if there were statistically significant differences between water parameters between each week. 4.3 Results 4.3.1 Sporulation All fragments were observed to be in a vegetative state prior to fragmentation (Figure 4.2, a). During the culturing of both the fragmented and whole sections of Ulva cellular changes were observed. Cells were seen to become displaced in preparation for gamete formation (Figure 4.2, b), and this was soon either followed by the formation of gametangia within the cells (Figure 4.2, c) or the cells returned to their initial position and remained vegetative. 50 a b 10 µm 10 µm dd c 10 µm ee 10 µm f 10 µm 10 µm Fig. 4.2 Thallus in vegetative state (a), Thallus with displaced chloroplasts (b) Thallus forming gametes (c) Thallus with fully formed gametes (d), Thallus after release of gametes (e), gametes swimming in Petri Dish (f). 51 If formation of gametangia were to occur they were observed to move around within the cells then exit through a pore in the cell leaving the cell empty and clear in colour (Figure 4.2 e). After the release of the gametangia (Figure 4.2 f) gametes were distinctively recognised by their elliptic shape and red eyespot, and they were observed swimming swiftly around the Petri dish propelled by two flagella. Data was found to significantly violate the requirement for repeated measures ANOVA as the Greenhouse Geisser epsilon was greatly smaller than one (0.3240). To compensate for possible effects of non-sphericity in the measurements compared this correction factor was applied to the ANOVA (Table 4.2). The ANOVA with these corrections showed a statistically significant interaction at the highest level Time, Species, Treatment and Week (P < 0.006). Table 4.2. Summary of repeated measures ANOVA with three grouping factors (Species, Treatment, Week) and one trial factor (Time) and their interaction effects on mean degree of sporulation in Ulva. A Greenhouse-Geisser epsilon of 0.3240 was applied to the degrees of freedom for all time effects and interactions. Source Species Treatment Week Species, Treatment Species, Week Treatment, Week Species, Treatment, Week Error Time Time, Species Time, Treatment Time, Week Time, Species, Treatment Time, Species, Week Time, Treatment, Week Time, Species, Treatment, Week Error (Time) Green house epsilon = 0.3240 df 2 1 3 2 6 3 6 48 Mean Square 7.30 4.01 3.21 2.89 1.51 1.87 0.99 0.67 F 10.83 5.96 4.76 4.30 2.25 2.78 1.47 34.58 p <0.001* 0.0018* 0.005* 0.019* 0.054 0.051 0.207 6 12 6 18 12 36 18 36 288 0.52 0.07 0.08 0.16 0.07 0.05 0.06 0.04 0.01 27.02 3.93 4.24 8.26 3.83 2.60 3.56 2.54 <0.001* 0.006* 0.0018* <0.001* 0.007* 0.005* 0.004* 0.006* * denotes statically significant P<0.05 52 As an experimental seaweed U. intestinalis was far superior to the other two species, demonstrating an average sporulation rate twice (0.48) that of U. lactuca (0.21) and more than six times greater than that of U. prolifera (0.07). All three species showed a similar trend in beginning sporulation 48 hrs after treatment. This tended to peak at 72 hrs post-treatment and stabilise after 72 hrs until the end of the experiment (264 hrs) (Figure 4.3). 0.7 0.6 Mean degree of sporulation 0.5 0.4 U. intestinalis 0.3 U. lactuca 0.2 U. prolifera 0.1 0 24 48 72 96 168 216 264 -0.1 -0.2 Time (hours) Fig 4.3 Time profile of mean sporulation between species, mean ± se (n=12) Fragmented thalli experienced a higher degree of sporulation over whole thalli. At the end of experimentation (264 hrs) fragmented thalli had achieved on average more than twice the degree of sporulation with an average of 0.41 in fragmented thalli and an average of only 0.19 in whole thalli (Figure 4.4). 53 0.5 Mean degree of sporulation 0.4 0.3 Fragmented 0.2 Whole 0.1 0 24 48 72 96 168 216 264 -0.1 Time (hours) Fig 4.4 Time profile of mean sporulation between treatments, mean ± se (n=36) U. lactuca and U. intestinalis were both found to respond positively to fragmentation, with greater sporulation rates in fragmented (0.42, 0.58) than whole (0.00, 0.38) thalli (Figure 4.5). Fragmentation was not found to improve sporulation in U. prolifera, where the average degree of sporulation was higher in whole thalli (0.11) than in fragmented thalli (0.02). 0.7 Average Sporulation 0.6 0.5 0.4 Whole 0.3 Fragmented 0.2 0.1 0 Species 3 U. intestinalis 2 U. lactuca 1 U. prolifera 0 4 Fig 4.5 Mean sporulation of fragmented and whole thalli in U. prolifera, U. lactuca and U. intestinalis 54 (n=84). Sporulation was observed to significantly fluctuate between weeks (Figure 4.6). The highest degree of sporulation was seen in week one with an average of 0.55 at the end of experimentation. This was followed by week two (0.33) and then week four (0.27). Week three experienced on average the least amount of sporulation with only a rate of only 0.05 at the end of experimentation. Water qualities remained very stable during the experiment (Figure 4.7). A one-way ANOVA performed showed that there was no significant difference between temperature (P> 0.05), salinity (P> 0.05), dissolved oxygen (P> 0.05) and pH (P> 0.05) between the four weeks. 0.7 0.6 Mean degree of sporulation 0.5 0.4 Week 1 Week 2 0.3 Week 3 Week 4 0.2 0.1 0 24 48 72 96 168 216 264 -0.1 -0.2 Time (hours) Fig 4.6 Time profile of mean sporulation between weeks, mean ± se (n=9) 55 40 Concentration 35 30 Temperature ºC 25 Salinity 20 Dissolved oxygen mg/L pH 15 10 5 0 0 1 2 3 4 5 Time Fig 4.7 Water parameter values at the start of each week, mean (n=3) 4.3.2 Fouling Culture dishes and or outdoor stock cultures were frequently observed to foul with marine phytoplankton, isopods, amphipods, copepods and marine flies (Figure 4.8). In total 17 fouling organisms were observed (See Appendix). Benthic epiphytes were observed to smother the thalli of Ulva thereby reducing its photosynthetic capacity, whilst isopods and amphipods were observed to feed directly on the tissue of the alga. a b Fig. 4.8 Culture dish fouling (a) and outdoor culture stock fouling (b). 56 4.4 Discussion The current experiment averaged zooid formation of less than 33%. Previous studies using similar techniques have achieved levels of up to 90% zooid formation (Hiraoka and Enomoto 1998). The low degree of sporulation achieved in the current experiment may be attributed to a number of factors. One of these factors may be that the algal material was primarily gametophytic. Previous work conducted by Hiraoka and Enomoto (1998) found differences in the degrees of sporulation between fragmented sporophytes and gametophytes, with 90% of sporophytes and only 40% of gametophytes becoming reproductively mature within three days. It is uncertain why this difference occurs but its is believed to be due to the differences in the system of zooid formation as sporophytes produce zoospores by meiotic and mitotic cell division, while gametophytes produce gametes only by mitotic cell division. Low sporulation levels could also be attributed to sporulation inhibiting substances released by thalli when fragmented. The active chemicals are more readily released from fragmented thalli than from whole plants (Neilsen and Nordby 1975). Keeping the fragment concentration low and effectively reducing the concentration of inhibiting substance can improve generative divisions of most cells. It is unknown if this affected the sporulation rates in the current experiment and this is an important aspect for further studies, particularly if the process is to be undertaken at large scale. Fragmentation generally improved sporulation rates with fragmented thalli on average having a higher degree of sporulation than whole thalli. This result is consistent with work conducted using U. mutabilis where fragmentation increased sporulation by 15.8 to 80% (Nordby 1977). 57 Formation of gametes in un-fragmented thalli is most likely a response due to the renewal of medium. Previous studies by Thaidens and Zeuthen (1967) have shown that a renewal of the culture medium can stimulate the formation of gametes in Ulva, presumably through changes in water parameters including pH, temperature and salinity. Another factor which could have been responsible for inducing sporulation in un-fragmented thalli is the fact that in order to obtain 1 g of U. lactuca the thalli had to be torn. This small amount of damage to the thalli may have induced some degree of sporulation in the un-fragmented experimental units. The differences in the degrees of sporulation between U. prolifera, U. intestinalis and U. lactuca may be due to the age of the plant material. Previous work conducted by Føyn (1955) found that sporulation is age dependant with plants under two months old seldom successfully releasing gametes and zoospores. In the current experiment it was believed that all of the algal material was mature and well over two months old however the exact age of the algal material was unknown and age may have contributed to some of the difference in the degree of sporulation between the three species that were tested. Another factor which has been found to result in a difference between sporulation is the number of cellular layers. Previous work conducted by Nordby (1977) stated that double-layer fragments tend to sporulate later than single-layered fragments. This finding was not observed in the current experiment with both single-layered fragments U. prolifera and U. intestinalis reaching reproductive maturity at the same time (48 hrs) as the double-layered fragments of U. lactuca. No experimental cultures were found to release gametes within two days of treatment. At least two days were needed for the completion of the maturation process in all three species. This result coincides with previous studies which found that other species such as U. spinulosa release their gametes after two to three days (Hiraoka et al. 2003). U. pertusa has been found to take four days 58 (Hiraoka and Enomoto 1998), U. mutabilis has taken two days (Nordby 1974, Nordby 1977), U. prolifera has taken three days (Hiraoka and Oka 2008), and various other Ulva sp. have taken two to five days (Lersten and Voth 1960). Nordby (1977) reported that the optimum pH for sporulation lies between 8.0 and 8.5. Outside this range the rate of sporulation was found to decrease. However earlier work conducted by Lersten and Voth (1960) found that a pH value of between 6.5 and 7.5 gave the highest degree of sporulation. In the current experiment the pH ranged from 7.67 to 8.01 at the time of transfer. Sporulation was observed in the current experiment to generally be higher when the pH was above 7.50, and a lower pH level appeared to possibly have a negative effect with the lowest weekly sporulation coinciding with the lowest pH. However these factors were not specifically tested. Macroinvertebrate grazers (amphipods and isopods) were frequently observed in outdoor stock cultures at BIRC. These cultures were approaching their useful life and were in need of renewal when these observations were made, but the organisms detected were indicative of the particular problems that would be faced by culturist’s at large scale. In more natural systems these macroinvertebrate grazers have been found to have less detrimental effects of macroalgae growth. Studies conducted by Kamermans et al. (2002) found that in the field the exclusion of grazing amphipods and isopods did not stimulate Ulva growth, in fact growth rates were observed to be higher in enclosures that allowed grazing. It was discovered that when epiphytic diatoms were present the amphipod Gammarus locusta and isopod Idotea chelipes grazed on the epiphytes and not on Ulva tissue. However, in natural systems wild macroinvertibrate populations are heavily regulated by predation, and mainly by fish (Holmlund et al. 1990). Interestingly, amphipods were noted to be difficult to find in tanks at BIRC where mullet and Ulva were both present, which suggests that such a co-culture approach could be useful at large scale in the future. 59 In managed algal cultures there are normally no predators of amphipods and isopods and so their population levels can build to very high unnatural levels with dire consequences for algal biomass. In the present work they were observed to quickly overtake culturing bowls, and this was minimised in experiments by thoroughly washing all of the seaweed biomass with freshwater to kill these grazers prior to use. This appeared to be effective with almost all amphipods and isopods dying within a few minutes of treatment. Unfortunately, this freshwater treatment was not found to be so effective at removing phytoplankton. Several laboratory cultures were observed to unintentionally become heavily fouled with phytoplankton. For the purposes of the study, these were discarded, but it raised additional concerns for similar problems that could be faced in commercial operations. Phytoplankton and macroalgae are well known to compete for both nutrients and light. This competition is the subject of many oceanographic studies, and an understanding of the processes at work provides the basis for pond management activities in large scale aquaculture. A good example of this relationship is provided by the annual model constructed to simulate the competition between U. rigida and phytoplankton in the Venice lagoon. This research concluded that while Ulva and phytoplankton constantly completed for available resources, Ulva is able to successfully persist because it can grow efficiently at lower light intensities (Solidoro et al. 1997). These results show that it is extremely important for all potential competitors and grazers to be removed from cultures. This will also apply to cultures being seeded with algal spores and germlings, since they are very delicate structures lacking the resistance mechanisms found in adult material (Lubchenco 1983). Physical stress, competition and grazing can also suppress early 60 development stages (Vadas et al. 1992). It would therefore be sensible for future studies to build stringent control measures into operating protocols. As observed in the current experiment reproductive cells can be easily obtained using the current fragmentation method. Swarmers and other induced propagules have been used for artificial seeding in the past which has lead to a simplification of procedures (Dan et al. 1997). With this method, reproduction is not restricted by season and potentially could be induced at any time under laboratory conditions, as long as there are well preserved thalli of the algae present. While sporulation was achieved in all three species through fragmentation the degree of sporulation was unfortunately considered to be too low for commercial application. Further work will therefore be needed to refining induction methods and achieve sporulation at larger scale. 4.5 Conclusions Sporulation was effectively induced in all three species of Ulva at BIRC. Levels were generally low and this could be attributed to a number of factors including the age of the algae material, the fact the algal material was primarily gametophytes or the algae material could have leaked sporulation inhibiting substances when fragmented. All of these factors have been previously found to reduce sporulation. Whilst the level of sporulation achieved would not be suitable for commercial application, this study provides a platform for future work in this area in the future. To improve future sporulation rates it could be worth looking at water flow through the fragmented material to reduce the amount of sporulation inhibiting substance, although the success of this approach would be difficult to monitor experimentally. It is recommended that further studies are conducted to improve the sporulation rates. 61 Chapter Five Summary 5.1 Concluding summary The objectives of the following study were to; identify all Ulva species naturally occurring at BIRC (i), establish the most optimum conditions for growing Ulva in the laboratory (ii), test if Ulva can be successfully induced into sporulation through a combination of fragmentation and renewal of medium and if so quantify the degree of sporulation and assess if it is suitable for a commercial application. This study achieved all of these objectives by identifying three species of Ulva, U. lactuca, U. intestinalis and U. prolifera to occur at BIRC. Establishing that Aquasol was the most favoured fertiliser for optimum growth and should be applied at a rate of 87 mg/L when Ulva is stocked at a density of 5-10 grams per litre and finally by establishing that Ulva in Southeast Queensland can be successfully induced into sporulation through a combination of fragmentation and renewal of medium. It was concluded however that sporulation levels achieved in the current experiment were too low for a commercial application and that future studies need to be undertaken to improve this rate. Studies could possibly look into other known inducers including dehydration, changes in salinity, nutrient, light and temperature as long as the methods are able to be reproduced on a mass scale. While the current study didn’t achieve sporulation levels desired for a commercial operation it did still play a vital role in contributing to improving our understanding of inducing reproduction of Ulva within Southeast Queensland. 62 References Back, S., Lehvo, A. and Blomster, J. 2000. Mass occurrence of unattached Enteromorpha intestinalis on the Finnish Baltic Sea Coast. Ann. Bot. Fennici. 37: 155-161. Balducci, C., Sfriso, A. and Pavoni, B. 2001. Macrofauna impact on Ulva rigida C. Ag. production and relationship with environmental variables in the lagoon of Venice. Mar. Enviro. Res. 52: 27-49. Bartoli, M., Nizzoli, D., Naldi, M., Vezzulli, L., Porrello, S., Lenzi, M. and Viaroli, P. 2005. Inorganic nitrogen control in wastewater treatment ponds from a fish farm (Orbetello, Italy): Denitrification versus Ulva uptake. Mar. Poll. Bull. 50: 1386-1397. Beach, K. S., Smith, C. M., Michael, T. and Shin, H. 1995. Photosynthesis in reproductive unicells of Ulva fasciata and Enteromorpha flexuosa: implications for ecological success. Mar. Ecol. Prog. Ser. 125: 229-237. Bold, H.C. and Wynne, M.J., 1985. Introduction to the Algae: Structure and Reproduction. Second Edition. Englewood Cliffs, New Jersey: Prentice-Hall, Inc. pp. 720. Bliding, C. 1963. A critical survey of European taxa in Ulvales. Part I. Capsosiphon, Percursaria, Blidingia, Enteromorpha. Opera Botantica a Societate Lundensi. 8: 1-160. Blomster, J., Maggs, C. A. and Stanhope, M. J. 1998. Molecular and morphological analysis of Enteromorpha intestinalis and E. compressa (Chlorophyta) in the British Isles. J. Phycol. 34: 319340. Braten, T. and Nordby, Ø 1973. Ultrastructure of meiosis and centriole behaviour in Ulva mutabilis Føyn. J. Cell Sci. 13: 69-8. Cater, N. 1926. An investigation into the cytology and biology of the Ulvaceae. In: Smith, G. M. 1947, On the reproduction of some pacific coast species of Ulva. Am. J. Bot. 84: 80-87. Christie, A. O. and Evans, L. V. 1962. Periodicity in the liberation of Gametes and Zoospores of Enteromorpha intestinalis Link. Nature. 193: 193-94. Corradi, M. G., Gorbi, G. and Zanni, C. 2006. Hypoxia and sulphide influence gamete production in Ulva sp. Aquat. Bot. 84: 144-150. Cribb, A. B. 1956. An ecological & taxonomic account of the marine algae of South-Eastern Queensland: II, unpublished thesis (PhD), University of Queensland. pp. 28-31. Dan, A., Ohno, M., Matuoka, M. 1997. Cultivation of the green alga Enteromorpha prolifera using chopped tissue for artificial seeding. In: Dan, A., Hiraoka, M., Ohno., M. and Critchley, A. T. 2002. Observations on the effect of salinity and photon fluence rate on the induction of sporulation and rhizooid formation in the green algae Enteromorpha prolifera (Müller) J. Agardh (Chlorophyta, Ulvales). Fish. Sci. 68: 1182-1188. 63 Dan, A., Hiraoka, M., Ohno., M. and Critchley, A. T. 2002. Observations on the effect of salinity and photon fluence rate on the induction of sporulation and rhizooid formation in the green algae Enteromorpha prolifera (Müller) J. Agardh (Chlorophyta, Ulvales). Fish. Sci. 68: 1182-1188. Davie, P. 1998. Algae. In: Wild guide to Moreton Bay, Queensland Museum, Brisbane. pp. 408. Dawson, E. Y. 1956. How to know seaweeds. Brown Company. Iowa. pp. 279. Dion, C. G., Noailles, M. C., Reviers, B. D., Fontaine, J. M., Berger-Perrot, Y. and Loiseaux-de Goe, S. 1998. Ulva armoricana (Ulvales, Chlorophyta) from the coasts of Brittany (France). II. Nuclear rDNA ITS sequence analysis. Eur. J. Phycol. 33: 81-6. FAO 2001. Production and utilization of products from commercial seaweeds. Electronic edition http://fao.org Fritsch, F. E. 1956. The structure and reproduction of the algae, Volume I. The Syndics of the Cambridge University Press, pp. 453. Fletcher, R. L. 1996. The occurrence of “green tides” – a review. In Schramm, W and Nienhuis, P. H., eds. Marine benthic vegetation in Europe: recent changes and the effect of eutrophication. Springer, Heidelberg, Germany. pp. 7-43. Foged, N. 1978. Diatoms in Eastern Australia. Bibliotheca Phycologia. Band 41. J. Cramer, Vaduz, Germany, pp. 242. Føyn, B. 1955, Specific Differences between Northern and Southern European Populations of the Green Alga Ulva latuca L. Pubbl. Staz. Zool. Napoli. 27: 261-270. Giordani, G., Azzoni, R., Bartoli, M.and Viaroli, P. 1997. Seasonal variations of sulphate reduction rates, sulphur pools and iron availability in the sediment of a dystrophic lagoon (sacca di Goro, Italy). Water Air Soil Poll. 99: 363-371. Greenhouse, S. W. and Geisser, S. 1959. On methods in the analysis of profile data. Psychometrika. 24: 95-112. Hayden, H. S., Blomster, J., Maggs, C. A., Silva, P. C., Stanhope, M. J. and Waaland, J. R. 2003. Linnaeus was right all along: Ulva and Enteromorpha are not distinct genera. Eur. J. Phycol. 38: 211-294. Han, T. and Choi, G. 2005. A novel marine algal toxicity bioassay based on sporulation inhibition in the green macroalgae Ulva pertusa (Chlorophyta). Aquat. Toxicol. 75: 202–212 Hasle, G. R. and Syvertsen, E. E. 1996. Marine diatoms. In Tomas, C. R. [Ed.] Identifying Marine Diatoms and Dinoflagellates. Academic Press, San Diego, pp. 5–385. Hiraoka, M. and Entomoto, S. 1998. The induction of reproductive cell formation of Ulva pertusa Kjellman (Ulvales, Ulvophyceae). Phycol. Res. 46: 199-203. Hiraoka, M. and Oka, N. 2008. Tank cultivation of Ulva prolifera in deep seawater using a new ‘germling cluster’ method. J. Appl. Phycol. 20: 97-102. 64 Hiraok, M., Shimada, S., Serisawa, Y., Ohno, M. and Ebata, H. 2003. Two different genetic strains of stakled-Ulva (Ulvales, Chlorophyta) grow on intertidal rocky shores in Ebisujima, central Japan. Phycol. Res. 51: 161-167. Hoffmann, A. J. and Ugarte, R. 1985. The arrival of propagules of marine macroalgae in the intertidal zone. J. Exp. Mar. Bio. Ecol. 92: 83-95. Holmlund, M. B., Peterson, C. H. and Hay, M. E. 1990. Does algal morphology affect amphipod susceptibility to fish predation? J. Exp. Mar. Biol. Ecol. 139: 65-83. Jiménez del Río, M., Ramazanov, Z. and García-Reina, G. 1996. Ulva rigida (Ulvales, Chlorophyta) tank culture as biofilters for dissolved inorganic nitrogen from fishpond effluents. Hydrobiologia. 326: 61-66. Jones, A. B, Preston, N. P., and Dennison, W. C. 2002. The efficiency and condition of oysters and macroalgae used as biological filters of shrimp pond effluent. Aquaculture Res. 33: 1-19. Kamermans, P., Malta, E. J, Verschuure, J. M., Lentz, L. F. and Schrijvers, L. 1998. Role of cold resistance and burial for winter survival and spring initiation of an Ulva spp. (Chlorophyta) bloom in a eutrophic lagoon (Veerse Meer lagoon, the Netherlands). Mar. Biol. 131: 45-51. Kamermans, P., Malta, E., Verschuure, J., Scrijvers, L., Lentz, L. and Lien, A. 2002. Effect of grazing by isopods and amphipods on growth of Ulva spp. (Chlorophyta). Aquat. Ecol. 36: 425433. Leal, M. C. F., Vasconcelos, M. T., Sousa-Pinto, I. and Cabral, P.S. 1997. Biomonitoring with benthic macroalgae and direct assay of heavy metals in seawater of the Oporto coast (northwest Portugal). Mar. Poll. Bull. 34: 1006-1015. Lersten, N. R and Voth, P. D. 1960. Experimental control of zooid discharge and rhizooid formation in the green alga Enteromorpha. Bot. Gaz. 122: 33-45. Lin, A., Shen, S., Wang, J. and Yan, B. 2008. Reproduction Diversity of Enteromorpha prolifera. J. Integr. Plant Biol. 50: 622-629. Linnaeus, C. 1753. Species plantarum. 2: 1163-1164. Lubchenco, J. 1983. Littorina and Fucus: effects of herbivores, substratum heterogeneity and plant escapes during successions. Ecol. 64: 1116-1123. Luning, K. and Pang, S. 2003. Mass cultivation of seaweeds: current aspects and approaches. J. Appl. Phycol. 15: 115-119. Luning, K., Kadel, P. and Pang, S. 2008.Control of reproductive rhythmicity by environmental and endogenous signals in Ulva pseudocurvata (Chlorophyta). J. Appl. Phycol. 44: 886-873. McArthur, D. M. and Moss, B. L. 1979. Gamtogenesis and gamete structure of Enteromorpha intestinalis (L.) Link. Br. Phycol. J. 14: 43-57. 65 Monterey Bay Aquarium Research Institute 2001. Biotic Interactions: Ulva ecology http://www.mbari.org/staff/conn/botany/greens/anna/frontpages/biotic.htm Mshigeni, K. E. and Kajumulo, A. A. 1979. Effects of the environment on polymorphism in Ulva fasciata Delile (Chlorophyta, Ulvaceae). Bot. Mar. 22:145-8. Nakanishi, K., Nishijima., M. Nishimura., M. Kuwano K. and Saga. N. 1996. Bacteria that induce morphogenesis in Ulva pertusa (Chlorophyta) grown under axenic conditions. J. Phycol. 32: 479482. National Academy of Agriculture Sciences, India 2003. Seaweed cultivation and utilisation. pp. 5. Naylor, R. L., Goldburg, R. J., Primavera, J. H., Kautsky, N., Beveridge, M. C. M., Clay, J., Folke, C., Lubchenco, J., Mooney, H. and Troell, M. 2000. Effect of aquaculture on world fish supplies. Nature. 405: 1017-1024. Neori, A., Chopin, T., Troell, M., Buschmann, A. H., Kraemer, G. P., Halling, C,. Shpigel, M. and Yarish, C. 2004. Integrated aquaculture: rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture. 231: 361: 391. Neori, A., Krom, M. D., Ellner, S. P., Boyd, C. E., Popper, D., Rabinovitch, R., Davison, P. J., Dvir, O., Zuber, D., Ucko, M., Angel, D. and Gordin, H. 1996. Seaweed biofilters as regulators of water quality in integrated fish- seaweed culture units. Aquaculture. 141: 183-199. Neori, A., Shpigel, M. and Ben-Ezra, D. 2000. A sustainable integrated system for culture of fish, seaweed and abalone. Aquaculture. 186: 279-291. Neori, A. 1996. The type of N-Supply (Ammonia or nitrate) determines the performance of seaweed biofilters integrated with intensive fish culture. Isr. J. Aquacult./Bamidgeh. 48: 19-27. Niesenbaum, R. A. 1988. The ecology of sporulation by the macroalgae Ulva lactuca L. (Chlorophyceae). Aquat. Bot. 32: 155-166. Nilsen, G. and Nordby, O. 1975. A sporulation-inhibiting substance from vegetative thalli of the green alga Ulva mutabilis, Føyn. Planta (Berl.). 125: 127-139. Nordby, Ø. 1974. Light microscopy of meiotic zoosporogenesis and mitotic gametogensis in Ulva mutabilis Føyn . J. Cell Sci. 15: 443-455. Nordby, Ø. 1977. Optimal conditions for meiotic spore formation in Ulva mutabilis Føyn. Bot. Mar. 20: 19-28. Nordby, Ø and Hoxmark, R. C. 1972. Changes in cellular parameters during synchronous meiosis in Ulva mutabilis Føyn. Exp. Cell. Res. 75: 321-328. Ohno, M. 1993. Cultivation of the green algae, Monostrama and Enteromorpha “Aonori”. In: Hiraoka, M. and Oka, N 2008. Tank cultivation of Ulva prolifera in deep seawater using a new ‘germling cluster’ method. J. Appl. Phycol. 20: 97-102. 66 Palmer, P. J., Burke, M. J., Palmer, C. J. and Burke, J. B. 2007. Developments in controlled greenwater larval culture technologies for estuarine fishes in Queensland, Australia and elsewhere. Aquaculture 272: 1-21. Palmer, P. J. 2008. Polychaete-assisted sand filters – prawn farm wastewater remediation trial. National Landcare Programme Innovation Grant No. 60945 Technical Report p. 61. Phillips, J. A. 1997. Algae. In: Queensland Plants: Names and Distribution. (Henderson, R.J.F. Eds), Queensland Herbarium, Department of Environment, Brisbane. pp. 286. Phillips, J. A. 1998. Field, Anatomical and Development Studies on Southern Australia Species of Ulva (Ulvaceae, Chlorophyta). Aust. Syst. Bot. 1: 411-456. Phillips, J. A. and Clayton, M. N. 1983. Morphology and Development in Culture of Enteromorpha linza (L.) J. Agardh (Ulvaceae, Chlorophyta), a Common Marine Alga of Southern Australia. Aust. Syst. Bot. 31: 11-18. Phillips, J. A. and McGregor, G. 2007. Protista - Algae. In: Census of the Queensland Flora (Bostock, P. D. and Holland, A. E. Eds), Queensland Herbarium, Environmental Protection Agency, Brisbane. pp. 298. Pickett-Heaps, J. D. 1975. Green Algae: Structures, Reproduction and Evolution in selected genera. University of Colorado. pp. 208-216. Pringle, J. D. 1986. Swarmer release and distribution of life-cycle phases of Enteromorpha intestinalis (L.) in relation to environmental factors. J. Exp. Mar. Biol. Ecol. 100: 97-111. Radmer, J. R. 1996. Algal diversity and commercial algal products. Bio. Sci. 46: 263-270. Round, F. E., Crawford, R. M. and Mann, D. G. 1990. The Diatoms: Biology and Morphology of the Genera. Cambridge University Press, Cambridge, pp. 747. Rowell, J. G. and Walters, R. E. 1976. Analysing data with repeated observations on each experimental unit. J. Agric. Sci. 87: 423-432. Santelices, B. and Ugarte, R. 1987. Algal life-history and resistance to digestion. Mar. Ecol. Prog. Ser. 35: 267-275. Sfriso, A. and Marcomini, A. 1996. Decline of Ulva growth in the lagoon of Venice. Bioresour. Technol. 58: 299-307. Sfriso, A., Pavoni, B., Marcomini, A. and Orio, A. A. 1992. Macroalgae, nutrient cycles, and pollutants in the Lagoon of Venice. Estuaries. 15: 517-528. Shimada, S., Hiraoka, M., Nabata, S., Lima, M., Masuda, M. 2003. Molecular phylogenetic analyses of the Japanese Ulva and Enteromorpha (Ulvales, Ulvophycease), with special reference to the free-floating Ulva. Phycol. Res. 51: 99-108. Silva, P. C. 1952. A review of nomenclatural conservation in the algae from the point of view of the type method. Univ. Calif. Publ. Bot. 25: 241-324. 67 Smith, G. M. 1947, On the reproduction of some pacific coast species of Ulva. A. J. Bot. 84: 8087. Sousa, A., Martins, I., Lillebo, A. I., Flindt, M. R., Pardal, M. A. 2007. Influence of salinity, nutrients and light on the germination and growth of Enteromorpha sp. Spores. J. Exp. Mar. Bio. Ecol. 341: 142-150. Solidoro, C., Brando, V. E., Fanco, D., Pastres, R., Pecenik, G. and Dejak, C. 1997. Simulation of the seasonal evolution of macroalgae in the lagoon of Venice. Environ. Model. Assess. 2: 65-71. Stafford, C. 1999. A Guide to phytoplankton of aquaculture ponds: Collection, analysis and identification. Queensland Department of Primary Industries, Brisbane, pp. 59. Stratmann, J., Paputsoglu, G. and Oertel, W. 1996. Differentiation of Ulva mutabilis (Chlorophyta) gametangia and gamete release are controlled by extracellular inhibitors. J. Phycol. 32: 1009-1021. Sze, P. 1997. Biology of the Algae. Third Edition, McGraw Hill. pp. 288. Tan, I. H., Blomster, J., Hansen, G. 1999. Molecular phylogenetic evidence for reversible morphogenetic switch controlling the gross morphology of two common genera of green seaweeds, Ulva and Enteromorpha. Mol. Biol. Evol. 16: 11-18. Thaidens, A. J. and Zeuthen, E. 1967. Meiosis and sporulation induced in sporophytes of Ulva mutabilis (“slender”) with synchronous mitosis. Planta (Berl.) 72: 60-65. Trainor, F. R. 1978. Introductory Phycology. University of Connecticut. John Wiley and Sons. pp. 525. Vadas, R. L., Johnson, S. and Norton, T. A. 1992. Recruitment and mortality of early postsettlement stages of benthic algae. Br. Phycol. J. 27: 331-351. Verlaque, M., Belsher, T. and Deslous-Paoli, J. M. 2002. Morphology and reproduction of Asiatic Ulva pertusa (Ulvales, Chlorophtya) in Thau Lagoon (France, Mediterranean Sea). Cryptogamie, Algol. 23: 301-310. Wang, H., Liu, C., Qin, C., Cao, S. and Ding, J. 2007. Using a macroalgae Ulva pertusa biofilter in a recirculating system for production of juvenile sea cucumber Apostichopus japonicus. Aquac. Eng. 36: 217-224. White, S. and Keleshian, M. 1994. A field guide to economically important seaweeds of Northern New England. University of Maine/University of New Hampshire Sea Grant Marine Advisory Program. MSG-E-93-16. http://www.noamkelp.com/technical/handbook.html Wikfors, G. H. and Ohno, M. 2001. Impact of algal research in aquaculture. J. Phycol. 37: 968974. Zemke-White, W. L and Ohno, M. 1999. World seaweed utilisation; An end-of-century summary. J. Appl. Phycol. 11: 369-376. 68 Appendix a b Zhong, Z. 1989. Marine planktology. China Ocean Press, Beijing, China, pp.454. 10 µm c 10 µm d 10 µm e 10 µm f 10 µm 10 µm g h 69 10 µm 10µm 10 µm i j 10 µm 10 µm k l 10 µm 10 µm m n 10 µm o 10 µm p 70 10 µm 10 µm r q 5 mm s 5 mm t 10 µm 10 µm v u 10 µm Appendix 1. Licmophora sp. (a), Cylindrotheca (b), Unknown sp. (c), Pleurosigma sp. (d), Unknown sp. (e) Oscillatoria sp. (f), Unknown sp. (g-p), Australian kelp fly pupae (q), Australian kelp fly (r), Unknown sp. (s) Unknown sp. (t) ,Navicula sp. (u) and amphipod (v). 71