Chapter 8: Reaction kinetics
advertisement

Reaction Kinetics
Main Textbook: Applied Physical Pharmacy
(Chapter 8)
Content
I.
II.
III.
IV.
V.
VI.
Rates and Orders of Reaction
Determination of The Order of Reaction
Stability and Shelf Life of Drugs
Other Factors Effecting The Rate of Reaction
Complex Reaction
Enzyme Catalysis Reaction
I. Rates and Orders of Reaction
I.1. Rate of reaction
Change of concentration as a function of time (dc/dt)
I.2. Order of reaction
Sum of the exponents (a + b + …)
aA + bB + … nN = Product
1 d [ A]
1 d [ B]
= − ⋅
= k[ A]a ⋅ [ B]b + ⋅ ⋅ ⋅[ N ]n (k : rate constant)
Rate = − ⋅
a dt
b dt
Ex. CH3COOH + C2H5OH
k
CH3COOC2H5 + H2O
d [CH 3COOH ]
d [C2 H 5OH ]
−
=−
= k[CH 3COOH ]1[C2 H 5OH ]1
dt
dt
∴ 1+1 = 2
Second order reaction
Molecularity
The number of molecules involved in elementary reaction,
not used in complex reactions
Ex) 2NO + O2
2NO2
2NO
N2O2
N2O2 + O2
2NO2
Specific rate constant
Rate constant in an elementary reaction
I.1. Zero - Order Reactions
Rate is independent of the concentration of the reactants
d [ A]
−
= k0
dt
At − A 0 = − k 0 t
⇔
⇔
∫
At
A0
A
t
t
dA = −K0 ∫ dt
0
=
A −k
0
0
t
⇔
c = a − kt
T1 / 2 =
1
2
k
A0
a
Concentration
Units for a zero - order reaction
d[ A] moles
=
k =−
dt
L⋅s
−k
Time
I.2. Pseudo - Zero Order Reactions
The concentration of drug remains constant for a period of time
Solid drug
saturated solution [A]
k
degraded drug [B]
d [ B]
−
= k[ A]
dt
A solid drug as a drug reservoir, and [A]n remains constant
d [ B]
= k app
dt
(Kapp = k[A])
I.3. First - Order Reactions
Rate of reaction is directly proportional to the concentration of
one of the reactants
d [ A]
−
= k[ A]
dt
Ex.
2H2O2
2H2O + O2
−
d [c ]
= k [c ]
dt
c = Concentration of H2O2 remaining undecomposition
t = Time
k = First - order velocity constant
The equation
LogC = LogC0 – kt/2.303
2 .303
log c 0
k=
t
C
Log
⇒
T 1/ 2 =
0.693
k
concentration
−
k
2.303
Units of first - order reaction
k=
d [ A] 1
moles / L
−1
⋅
=
=s
dt [ A] s ⋅ moles / L
0
Time
I.4. Pseudo - First Order Reactions
Consider a reaction of A and B forming produce C
A+B
k
C
− d [ A]
= k[ A]1[ B ]1
Overall, it appears to be second - order :
dt
If [B] ≫ [A] ; [B] is constant because so little [B] is used up in the
reaction
− d [ A]
= k app [ A]
dt
Ex. CH3COOH + Cl2
k
CH2ClCOOH + HCl
d [CH 3COOH ]
Rate = −
= k[CH 3COOH ][Cl2 ] = k 1[CH 3COOH ]
dt
I.5. Second - Order Reactions
The rates depend on the concentration of two reactants
[A] + [B] k C
− d [ A] − d [ B]
=
= k[ A][ B]
dt
dt
a, b = The initial concentration of A, B
dx
= k (a − x)(b − x)
x = The concentration each species reacting
dt
t = Time
When A = B
2
dx
a=b, the rate of reaction is :
= k (a − x)
dt
x
= kt
a(a − x)
k =
1 ⎛ x ⎞
⎜
⎟⇔
at ⎝ a − x ⎠
t
1/ 2
=
1
ak
x
a (a − x)
k
The units for second - order reaction
d[ A] 1
moles/ L
L
k=
⋅ 2=
=
dt A s(moles/ L)2 s ⋅ mole
Time
II. Determination of the Order of
Reaction
II.1. Graphic Method
The reaction is zero - order if a straight line results
when concentration C is plotted against t
The reaction is first - order if log(a - x) vs. t yields a straight line
The reaction is second - order if 1/(a - x) vs. t give a straight line
Plot of tertiary buty l alcohol
concentration against time
0.75
100
0.7
80
60
40
A plot of log concentration against time
2
lo g (c )
120
ln{ a/ (a- x)}
C o nc.
Decomposition of Tertiary butyl
alcohol with Sulfuric acid at 200C
0.65
0.6
1.75
1.5
0.55
20
1.25
0.5
0
0
10
20
30
Time, min
40
50
0
20
Time, min
40
60
0
20
Time, min
40
60
II.2. Half – Life Method for Determination
of Order
The half - life can be expressed in general terms
t1 / 2 = k
1
a ( n −1)
Zero - order = T1/2 proportional to initial concentration
First - order = T1/2 independent of concentration
Second - order = T1/2 proportion to the reciprocal of concentration
If two reactions are run at difference initial concentration a1, a2
the half life t1/2(1), t1/2(2)
a=
log(t1 / 2(1) / t1 / 2( 2) )
log(a2 / a1 )
+1
If a reaction is first - order
t1/2(1) = t1/2(2) ; log( t1/2(1) / t1/2(2) ) = log1 = 0
∴ a= 0+1=1
III. Stability and Shelf of Drugs
Shelf life is indicated by T90
[At] = Concentration after time t
[A0] = Initial concentration
[At] = [A0] – kt90
Ex. A drug contained 1.2 mg/ml. To obtain 90% of 1.2mg/ml :
1.2 mg/ml x 0.9 = 1.08 mg/ml
And
K=0.03 mg/ml/h
1.2 – 1.08 = 0.12
T
=
90
0.12
= 4h
0.03
Long-term/Accelerated Testing Conditions
Conditions
Minimum time period at
submission
Long-term testing
25±2℃,
60%RH±5%
12 months
Accelerated testing
40±2℃,
75%RH±5%
6 months
III.1. Accelerated Stability Testing
The first - order reaction
2.303
t=
log c0
k
C
Ex.6. The initial conc. : 200mg/ml
k= 3 x 10-5h-1 at 25℃. The limit of viability : 150mg/ml
Shelf life = ?
t=
2.303
200mg / ml
log
⋅
= 95,000h ≈ 1.1 year
−5 −1
3 ×10 h
150mg / ml
III.2. The Effects of Temperature
The Arrhenius equation can be used to indicate the effect of
temperature on the specific rate constant for reaction
k = Ae− Ea / RT
log k = log A −
Ea
2.303 ⋅ RT
Log A
Slope =
k = Specific reaction rate constant
A = Arrhenius factor
Ea = Energy of activation
R = Gas constant (1.987 cal/deg mole)
T = Absolute temperature
− Ea
2.303R
Log k
1
T
y2 − y1
Ea =
⋅ 2.303R
x2 − x1
Ea also can be obtained by considering ;
For a temperature T2 and T1
log
k2
Ea ⎛ (T2 − T1 ) ⎞
⎟⎟
=
⋅ ⎜⎜
k1 2.303R ⎝ T2T1 ⎠
Ex.8. T1= 383°K, k1= 2.0h-1 ; T2= 423°K, k2 = 3.8h-1
Ea = ? ; A = ? (R=1.987 cal deg-1 mol-1)
Solution :
Ea ⎛ 423 − 383 ⎞
1
3 .8
=
log
⎜
⎟.
2 .0
2 . 303 ⎝ 423 × 383 ⎠ 1 . 987
∴ Ea = 5.164 × 103 cal ⋅ mole −1
3
−
5
.
164
×
10
log( 5.5 × 10 − 4 s −1 ) = log A ⋅
⇒ A = 4.8 × 10 −1 s −1
2.303 × 1.987 × 383
III.3. Influences of pH on Reaction Rates
The magnitude of the rate of a hydrolytic catalyzed by
H+ and OH- ions can vary considerably with pH
- The H+ ion catalysis predominates at a lower pH
- The OH- ion catalysis operates at a higher pH at an intermediate
pH : The rate may be pH-independent or catalyzed by both H+
and OH- ions
- The pH of optimum stability
can be determined by plotting
log k against pH
Log k
pH, optimum stability
- The point of the reflection
of such a plot represents
the pH of optimum stability
pH
III.4. Effects of Solvents on Reaction
Rates
To stabilize the drug
- Alter the activity coefficients and transition state
- Change physicochemical parameter : pKa, surface tension, viscosity..
Less polar
A
Less polar
B
More polar
∆pH
III.5. Electrolyte Solutions and The
Dielectric Constant
The dielectric constant (E) of the solvent can influence
the rate of constant
k Za⋅Zb
log k = log ke∞ −
E
K : Rate constant for reaction
Ke∞ : Rate constant in a solvent of an infinite dielectric constant
Za : Charge on ion
Zb : Charge of a drug molecule
Opposite
If both the drug and solvent ions have
a similar charge, the slope is negative.
So drug in solvents with low E will
decrease the decomposition rate.
no stabilization with low E
-
If the charges on the attacking ion
and the drug molecules are opposite,
the slope of the plot is positive.
logk
Similar
stabilization
charge
with low E
-
(H2O)
(C6H6)
1
E
Log k against reciprocal values of
the dielectric constant for benzene
IV. Other Factors Effecting the
Rate of Reaction
IV.1. Presence of Additives
- Buffer salt
An increasing salt concentration can effect pKa
change in rate
constants
Drug degradation is promoted by general acid or base catalysis
- Addition of surfactant
Accelerate degradation because of micelle catalysis and charge
neutralization
Stabilize the drug by modifying the surface charge
IV.2. Modes of Pharmaceutical
Degradation
- Hydrolysis : H+ and OH- ions are the most common catalysts
- Oxidation : For reactions with atmosphere O2
• Drugs effected by O2 are dissolved in solvent
• Free radical mechanisms and chain reaction
- Photolysis : Degradation of a drug by light
Protection from Oxidation
+ Ascorbic acid is an electron donor
Poly- hydroxy phenolic compounds provide resonance stabilization
Sodium sulphite, metabisulfite, and bisulfate
+ Oil systems include tocopherol, ascorbyl palmitate…
+ Chelating agents include Cu2+, Fe2+, and Fe3+
+ Removal of O2, replace with N2 or CO2
+ Optimum pH
+ Protection from light
+ In regard to refrigeration, as temperature increases, oxidation reactions
increased
+ Surfactants aid surface protection and charge neutralization
IV.3. Stabilization
Protection also can be afforded by
- Selection of optimum pH
- Buffers
- Solvents
- Complexing agents
- Surfactants allow the incorporation of a drug into micelles to reduce
hydrolytic rate
- To formulation a drug as a suspension, the rate of breakdown is only
proportional to the concentration of the drug in solution
- H2O can be removed by freeze drying
- Chemical modification
V. Complex Reaction
V.1. Reversible Reaction
[A]
kf
kr
[B]
kf, kr : Rate constants
- The rate equation for this first - order reversible reaction is
A0 − Aeq
2.303
t=
⋅ log
A − Aeq
(k f − k r )
− d [ A]
= k f [ A] − k r [ B ]
dt
If it is plotted against log
A0 − Aeq
A − Aeq
2 . 303
; Slope =
k f − kr
V.2. Parallel Reaction
The rate equation for first - order of the parallel type
− d [c ]
= (k1 + k 2 )[C ] = kexp [C ]
dt
k1
k1, k2 : The rate constant for reactions A, B
Kexp : The rate constant found from experiments
C
k2
K1, k2 can be found by finding the ratio R= [A]/[B]
[ A] k1
R=
=
[ B] k 2
⎛ R ⎞
k1 = k exp ⎜
⎟
⎝ R + 1⎠
;
A
k2 =
k exp
( R + 1)
B
V.3. Consecutive Reaction
A
k1
B
k2
C
Where each step follows first - order rules
− d [ A]
= k1 [ A]
dt
The rate of change of decomposition of B is
− d [ A]
= k1 [ A] − k 2 [ B ]
dt
− d [ A]
= k2[B]
And that of C :
dt
Integration of equation : [A] = [A0]e-k1t
k1 [ A0 ] − k1t
[ B] =
.[e − e − k 2t ]
k 2 − k1
and [C] = [A0] - [A] - [B]
⎡
1
− k1t
− k 2t ⎤
= [A0 ]× ⎢1 +
(k 2 e − ke )⎥
⎣ k1 − k 2
⎦
The equations can be used to find : k1, k2 and [C]
VI. Enzyme Catalysis Reaction
Effect of substrate concentration on the rate of an enzyme
catalyzed reaction
[S] : Substrate concentration
V0 : Initial velocity
Km : The Michaelis-Menten
constant
V
max
Vmax
2
V0
km
[S]
E+S
k1
k2
ES
k3
k4
E+P
V0 is equal to the rate of breakdown of ES
The first - order rate equation : V0 = k3 [ES]
The second - order equation is utilized
d [ ES ]
= k1 ([ ET ] − [ ES ]) ⋅ [ S ]
dt
ET : Total enzyme concentration
K1 : Second order rate constant
From equation
− d [ ES ]
= k 2 [ ES ] + k 3 [ ES ]
dt
In the steady state condition
k1([ET] – [ES])[S] = k2[ES] + k3[ES]
[ S ]([ ET ] − [ ES ]) k 2 + k 3
=
= km
[ ES ]
k1
[ ES ] =
[ ET ][ S ]
K m + [S ]
V0 = k3
[ E T ][ S ]
km + [S ]
When the enzyme is saturated with substrate : Vmax = k3[ET]
V max ⋅ [ S ]
The Michaelis - Menten equation : V 0 =
km + [S ]
km 1
1
1
=
⋅
+
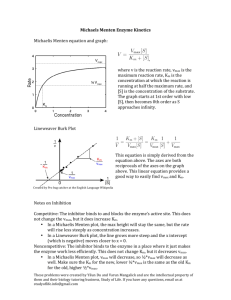
The Lineweaver - Burk equation :
V0 Vmax [ S ] Vmax
1
1
When
is plotted against
V0
[S ]
1
V0
A straight line is obtained
Slope =
km
Slope =
Vmax
Vmax and km can be obtained
1
Vmax
−1
km
1
[S ]
km
Vmax
References
Applied physical pharmacy
Physical pharmacy (Martin; 4 th)
물리약학 (제3판)
Drug stability (3 th)