Natural Gas Properties
advertisement

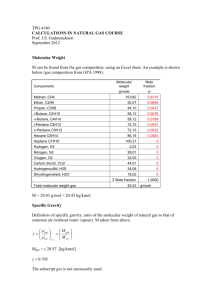
TPG 4140 NTNU Natural Gas Properties Professor Jon Steinar Gudmundsson Department of Petroleum Engineering and Applied Geophysics Norwegian University of Science and Technology Trondheim September 18, 2013 Outline • • • • • • • • • • Properties used in natural gas calculations Phase diagrams and terminology (CCP & CCT) Real gas law, z-factor, density and FVF Corresponding states Compressibility factor (z-factor) Example calculation (GPA gas composition) Heat capacity and heat capacity ratio Viscosity of natural gas Calorific values (GCV & NCV) Summary Properties Used – – – – – Density, need z-factor and molecular weight Flow in wells, need z-factor and viscosity Pressure drop in pipelines, need density and viscosity Temperature in pipelines, need heat capacity Compressor power and exhaust temperature, need molecular weight and heat capacity ratio – Molecular weight, need relative density (gravity) – Reynolds number, need density and viscosity – Wobbe index, need gross calorific value and relative density Calculations and Data on Home Page PHASE DIAGRAM Pedersen et al. (1989) Properties of Oils and Natural Gases, Gulf Publishing Company Rojey & Jaffret (1997) Rojey & Jaffret (1997) TERMINOLOGY • Natural gas, C1-C5+, water, inert gases • • • • NGL (Natural Gas Liquids), under pressure LPG (Liquefied Petroleum Gas), propan+butan, -42 C LNG (Liquefied Natural Gas), -162 C, 1 atm CNG (Compressed Natural Gas), 180-200 bar • Condensate (liquid), C4-C7, transition gas-to-oil • Oil, C6 and heavier fractions TYPICAL SPECIFICATIONS • Transport Specification – – – – Hydrocarbon dew point, 5-10 C below ambient Water dew point, about 5 C below HC dew point Temperature, 30-50 C Pressure, depends on receiving terminal • Sales Specification (in addition to above) – Heating value (GHV = Gross Heating Value), MJ/Sm3 – Wobbe Index (WI = GHV/(specific density)0,5 – Removal of non-HC gasser (inert gases) http://www.ipt.ntnu.no/~jsg/undervisning/prosessering/forelesninger/05-Produktspesifikasjoner.pdf TYPICAL SPECIFICATIONS COMPOSITION Generalization • Non-Associated (dry gas) • Associated gas (wet gas) methane > 90 volume % methane < 90 volume % • Søtt gass (sweet gas) • Surt gass (sour gas) CO2 < 2 volume % CO2 > 2 volume % • Søtt gass (sweet gas) • Surt gass (sour gas) H2S < 1 volume % H2S > 1 volume % Rojey & Jaffret (1997) fra Valais (1983) Volume Rate at s.c. to Mass Rate • • • • • • • • Compress 7 MSm3/d from 80 to 160 bara Inlet temperature 30 C Molecular weight M = 20.43 kg/kmol Atm. Pressure 1.01325 bara At s.c., ρ = 0.864 kg/Sm3 (T = 15 C) Mass flow rate 7∙0.864 = 6.05∙106 kg/d Per second m = 70 kg/s M and ρ from Excel sheets Real Gas Law pV znRT pv zRT z sc 1 p Tsc 1 Vsc V psc T z p Tsc 1 qsc q psc T z Density and FVF pV znRT M V n pM zRT V m3 3 B FVF Vsc Sm T psc z B Tsc p q qsc B Finding Real Gas Factor (z-factor) – Diagram based on corresponding states, reduced pressure and temperature for single components and pseudo-reduced pressure and temperature for natural gas. – Empirical equations matched to z-factor diagram for natural gas. Uses many constants and coefficients and in some cases iteration. – Equation Of State (EOS) such as Peng-Robinson, Redlich-Kwong and Benedict-Webb-Rubin. Implemented in many different computer programs. – EOS implemented in many commercial computer packages such as Hysys, Prosper and PVTsim. Real Gas Factor vs. Pressure at 25 C Fletcher (1993) Real gas factor with reduced p & T Fletcher (1993) Reduced p & T p pr p pr pc T Tr T pr Tc pc pci yi i Tc Tci yi i Kay’s Rule Corresponding States – When pressure and temperature are normalized using critical pressure and temperature, then all properties become the same/similar, irrespective of composition. – Normalized pressure or temperature are called reduced pressure or temperature in one component systems. – Normalized pressure or temperature are called pseudo-reduced pressure or temperature in multicomponent systems. – Commonly used when gas properties (natural gas and other gases) are to be correlated and/or presented. GPA (1998) Rojey o.a. (1997) Equations and Gravity p pc 4,892 0,405 ( MPa ) Tpc 94,72 170,75 ( K ) M gas Mair M gas 28,964 (at s.c.) Thomas o.a. (1970), fra Rojey o.a. (1997) Physical Constants of Natural Gas Campbell (1984) Example Calculation for Natural Gas GPA (1998) GPA (1998) Gas Composition (Excel sheet) Components Molecular weight Mole fraction Tci Tci Pci Pci g/mole yi oR K psia Mpa Methan, CH4 16,042 0,8319 343 190 667,8 4,61 Ethan, C2H6 30,07 0,0848 549,8 305 708 4,88 Propan, C3H8 44,10 0,0437 665,7 370 616 4,25 i-Butane, C4H10 58,12 0,0076 734,7 408 529 3,65 n-Butane, C4H10 58,12 0,0168 765 425 551 3,80 i-Pentane C5H12 72,15 0,0057 829 460 491 3,39 n-Pentane C5H12 72,15 0,0032 845 469 489 3,37 Hexane C6H14 86,18 0,0063 913 507 437 3,01 Heptane C7H16 100,21 0 972 540 397 2,74 Hydogen, H2 2,02 0 60 33 187 1,29 Nitrogen, N2 28,01 0 227,4 126 492 3,39 Oxygen, O2 32,00 0 277,8 154 731 5,04 Carbon dioxid, CO2 44,01 0 547,6 304 1071 7,38 Hydrogensulfid, H2S 34,08 0 672,4 373 1306 9,01 Dihydrogenoksid, H2O 18,02 0 1165 647 3199 22,06 Σ Mole fraction Total molecular weight gas 20,43 1,0000 g/mole Phase Envelope for GPA (1998) Gas Beggs (1984) Campbell (1984) Heat Capacity C p T T 2 Cp R A BT CT 2 Cp 1,702 9,08110 3 T 2,164 10 6 T 2 R CH 4 R 8,314 (kJ / kmol.K ) C p CH 4 0,2047 1,092 10 3 0,2603 10 6 (kJ / kmol.K ) Når per mol bruk molfraksjon for blanding Når per masse bruk massefraksjon for blanding Smith o.a. (1996) Heat Capacity Natural Gas Temperature, Pressure, Relative Density a = 0.90 b = 1.014 c = -0.700 d = 2.170 e = 1.015 f = 0.0214 Moshfeghian, M. (2013) C p a b T d e p 0.60 T c p f 0.025 Heat Capacity Ratio • • • • • • • • M = 20.43 kg/kmol T = 30 C = 86 F p = 80 bara k = 1.26 (from diagram) R = 8.314 kJ/kmol.K z = 0.783 k = 1.26 (from “old” equation = ideal gas) k = 1.19 (from “new” equation = real gas) Ratio of Specific Heat Capacity k k Cp Cv Cp C p zR k Cp Cp R H k U Heat Capacity Ratio Hysys Estimation GPA (1998) Associated Gas Comparison of Heat Capacity Ratio GPA (1998) Gas at 80 bara & 30 C k Diagram Ideal gas Ideal gas with z-correction Hysys ideal gas Hysys 1,26 1,26 1,19 1,158 1,714 Ideal Gas Compression m (kg/s) 70 M (kg/kmol) 20,43 R (J/kmolK) 8314,34 T innløp C 30 k (Cp/Cv) 1,26 p innløp bara 80 p utløp bara 160 P (MW) 6,44 T utløp C 77 k 1 k k p2 m 1 Pideal RT1 M k 1 p1 Real k Gas Compression m (kg/s) 7 M (kg/kmol) 20,43 R (J/kmolK) 8314,34 T innløp C 30 k (Cp/Cv) 1,19 p innløp bara 80 p utløp bara 160 P (MW) 6,33 T utløp C 65 k 1 k k p2 m 1 Pideal RT1 M k 1 p1 Viscosity from Diagram – Diagram shows viscosity against temperature for gas components (methane, ethane, propane etc.) at atmospheric pressure. – Empirical equation (shown under, based on kinetic theory of gases) gives estimate of viscosity to natural gas (mixture of methane, ethane, propane etc.) at atmospheric pressure. – Diagram gives viscosity ratio to viscosity at atmospheric pressure against reduced pressure and temperature 1/ 2 y M i i i 1/ 2 y M i i Katz o.a. (1959), fra Rojey o.a. (1997) Katz o.a. (1959), fra Rojey o.a. (1997) Viscosity Correlation – Several correlations in literature, for example Carr et al. (1954), Lee et al. (1966) and Pedersen et al. (1987). – Lee et al. (1966) correlation has recently been evaluated by Jeje and Mattar (2004) and has the form shown below. K, X and y are given by empirical equations. – The correlation is available as spreadsheet. K exp X y Combined Cycle Power Plant Combustion and Calorific Value Summary – Several physical and thermodynamic properties of natural gas are used in natural gas calculations. – Real gas law and reduced pressure and temperature used in diagrams to obtain z-factor. – Empirical correlations used for transport properties, for example for viscosity. – Heat capacity at constant pressure can be obtained from figures and equations. Also as function of pressure, temperature and relative density. – Heat capacity ratio values uncertain. – Equation Of State (EOS) used in computer programs for pVT properties (also thermodynamic properties). References – – – – – – – – – Beggs, H.D. (1984): Gas Production Operations, OGCI Publications, Tulsa, Oklahoma Campbell, J.M. (1994): Gas Conditioning and Processing, Campbell Petroleum Series, Norman, Oklahoma. Fletcher, P. (1993): Chemical Thermodynamics, Longman, Harlow, Essex. Gas Processors Association (1998): Engineering Data Book, Tulsa, Oklahoma. Gupta, N. (2011): Overview of Ormen Lange Project, Guest Lecture, TPG 4140 Natural Gas, NTNU. Jeje. O. & Mattar, L. (2004): Comparison of Correlations for Viscosity of Sour Natural Gas, 5th Canadian International Petroleum Conference, Calgary, Alberta, June 8-10, Paper 2004-214. Moshfeghian, M. (2013): Variation of Natural Gas Heat Capacity with Temperature, Pressure and Relative Density, J.M. Campbell & Co., (Internett February 2013). Rojey, A. (1997): Natural Gas, Éditions Technip, Paris. Smith, J.M., Van Ness, H.C. & Abbott, M.M. (1996): Introduction to Chemical Engineeering Thermodynamics, McGraw-Hill, New York.